Batch Stoichiometric Table Species Symbol A A B
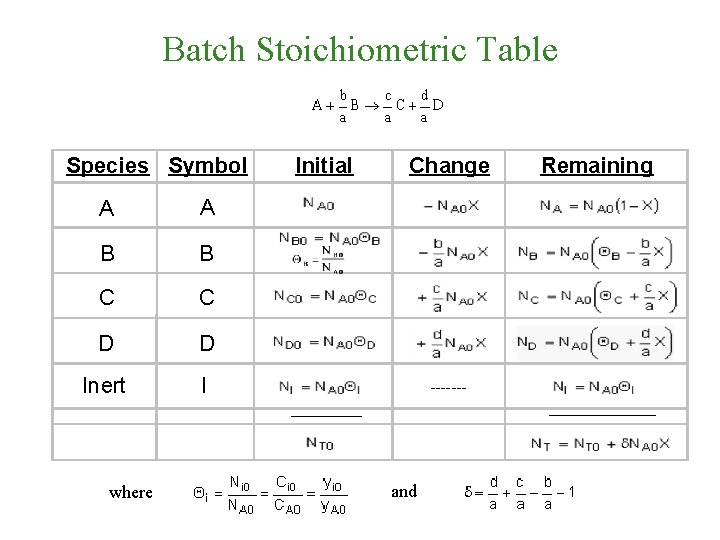
Batch Stoichiometric Table Species Symbol A A B B C C D D Inert I Initial Change ------______ where Remaining and
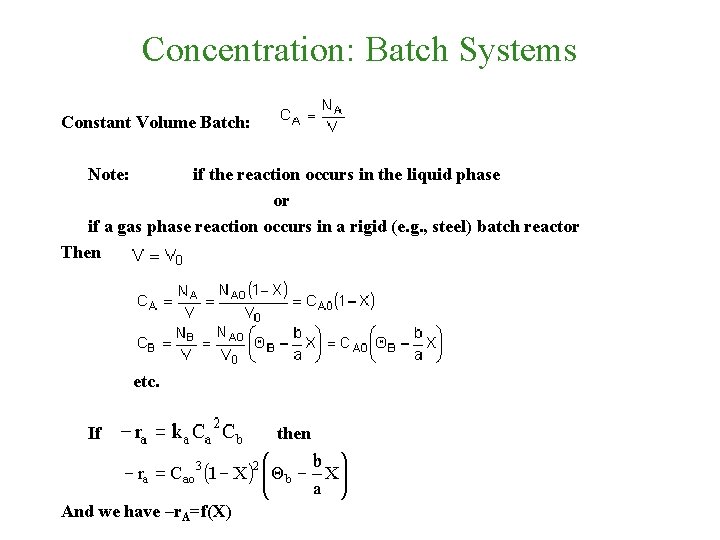
Concentration: Batch Systems Constant Volume Batch: Note: if the reaction occurs in the liquid phase or if a gas phase reaction occurs in a rigid (e. g. , steel) batch reactor Then etc. If And we have –r. A=f(X) then
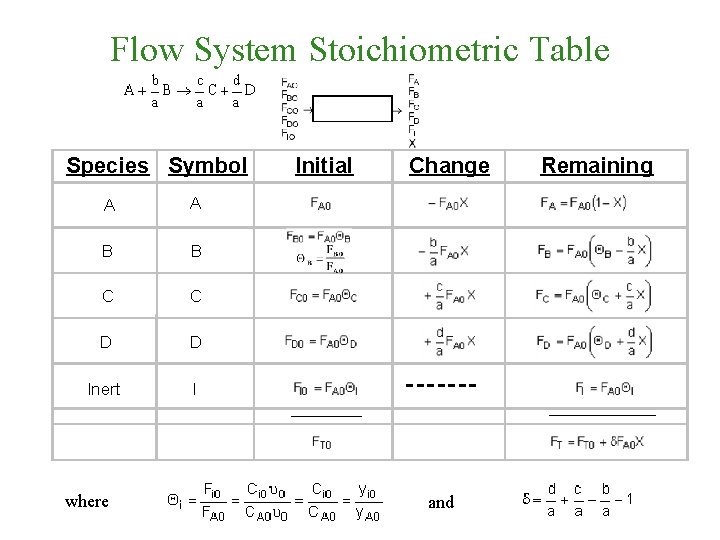
Flow System Stoichiometric Table Species Symbol A A B B C C D D Inert I Initial Change ______ where Remaining and
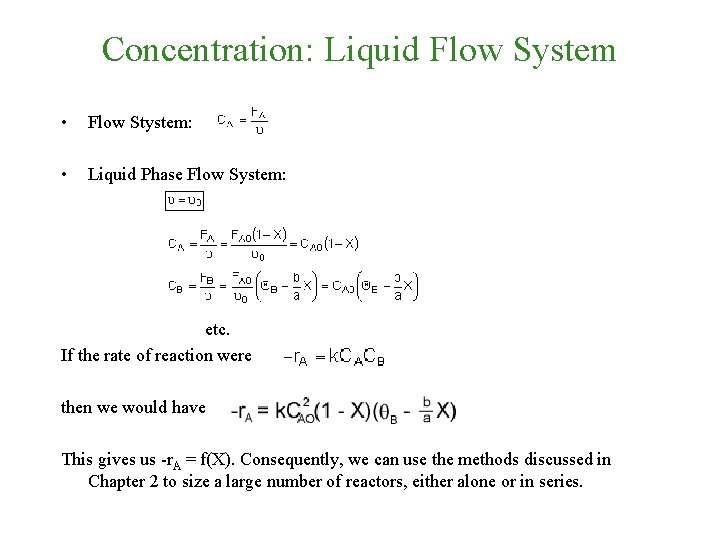
Concentration: Liquid Flow System • Flow Stystem: • Liquid Phase Flow System: etc. If the rate of reaction were then we would have This gives us -r. A = f(X). Consequently, we can use the methods discussed in Chapter 2 to size a large number of reactors, either alone or in series.
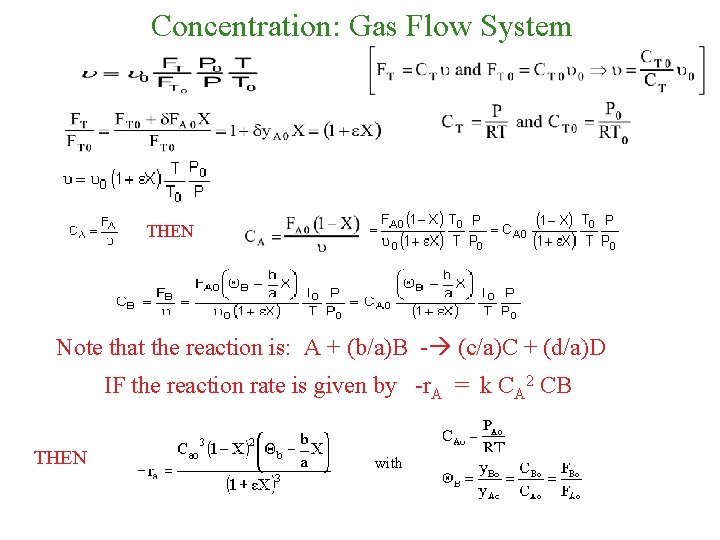
Concentration: Gas Flow System THEN Note that the reaction is: A + (b/a)B - (c/a)C + (d/a)D IF the reaction rate is given by -r. A = k CA 2 CB THEN with
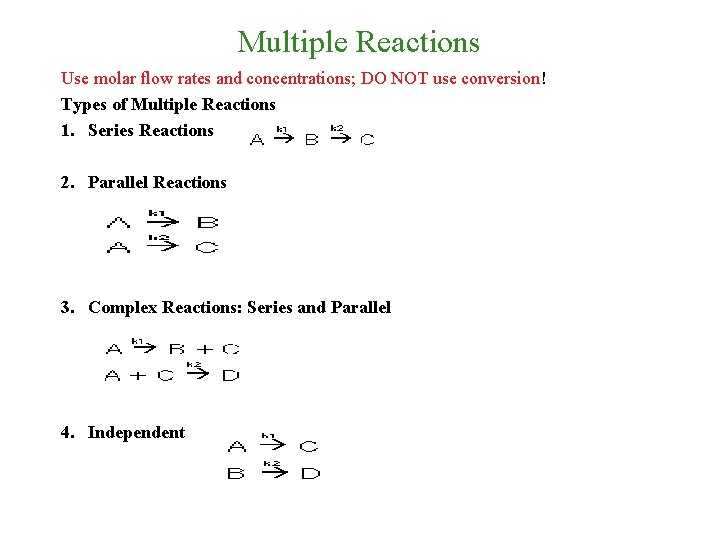
Multiple Reactions Use molar flow rates and concentrations; DO NOT use conversion! Types of Multiple Reactions 1. Series Reactions 2. Parallel Reactions 3. Complex Reactions: Series and Parallel 4. Independent
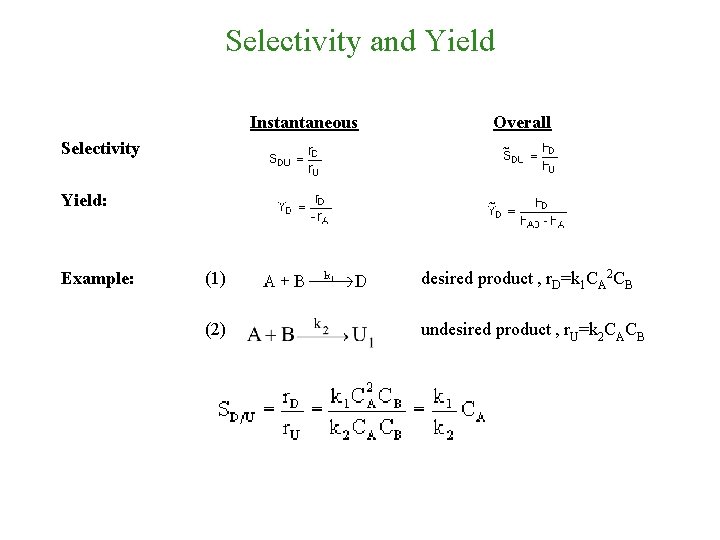
Selectivity and Yield Instantaneous Overall Selectivity Yield: Example: (1) desired product , r. D=k 1 CA 2 CB (2) undesired product , r. U=k 2 CACB
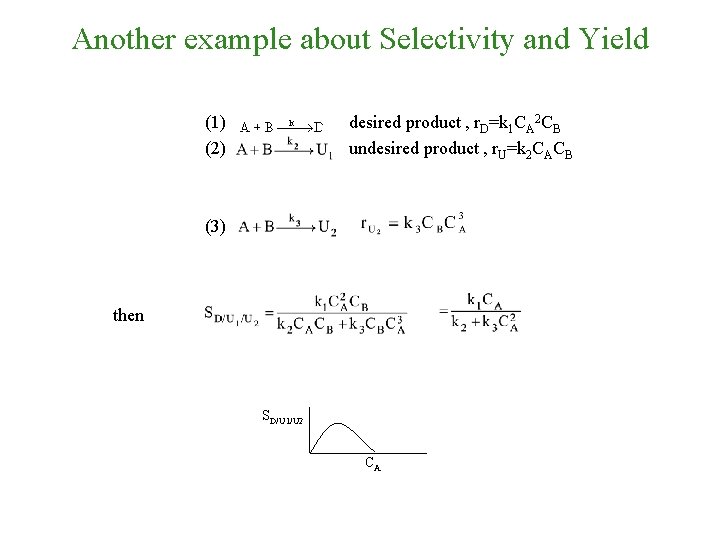
Another example about Selectivity and Yield (1) (2) desired product , r. D=k 1 CA 2 CB undesired product , r. U=k 2 CACB (3) then SD/U 1/U 2 CA
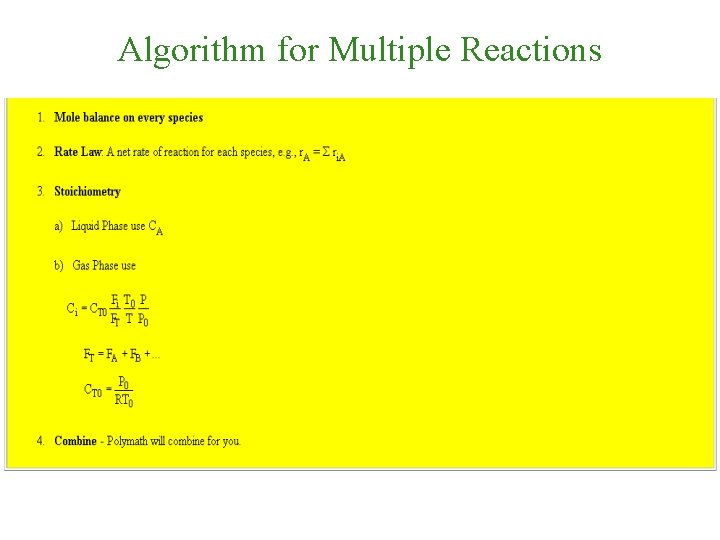
Algorithm for Multiple Reactions
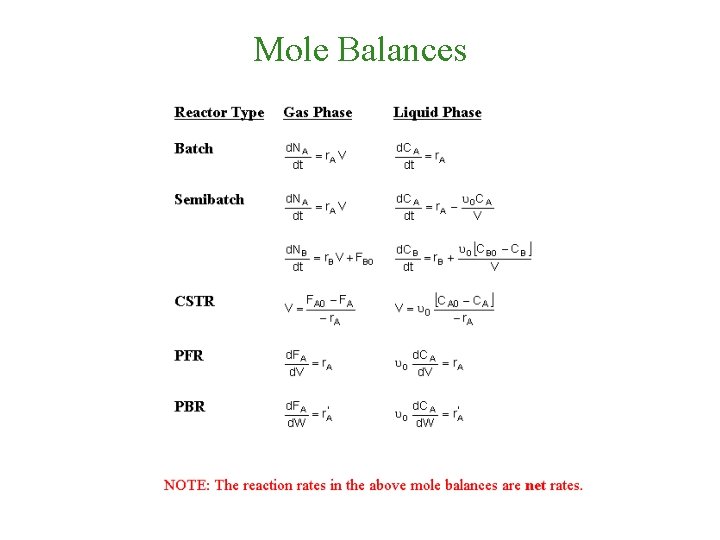
Mole Balances
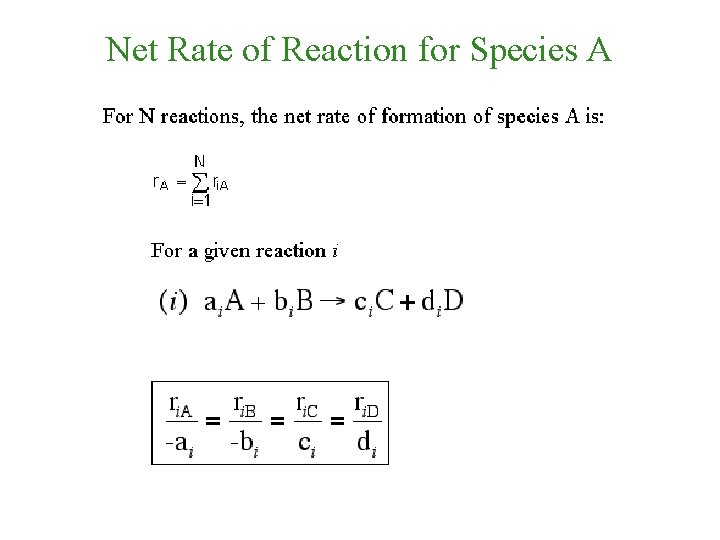
Net Rate of Reaction for Species A
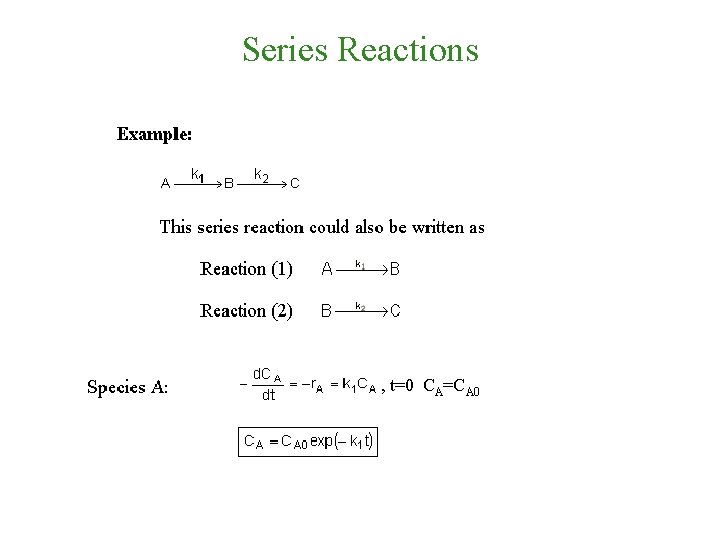
Series Reactions , t=0 CA=CA 0
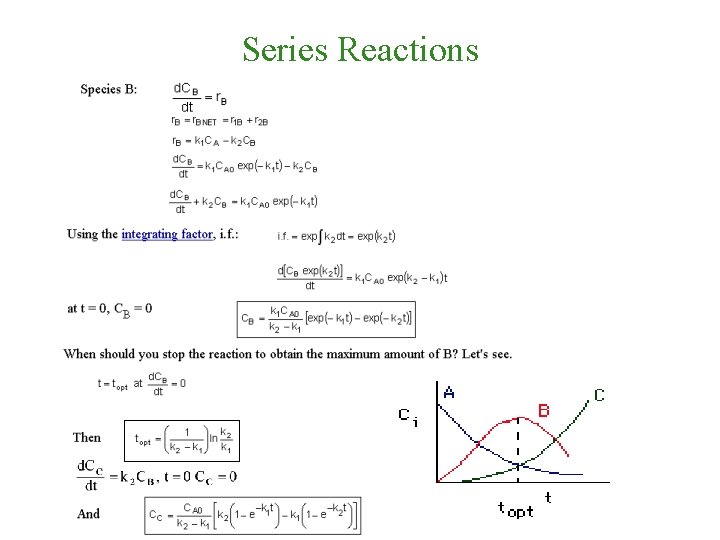
Series Reactions
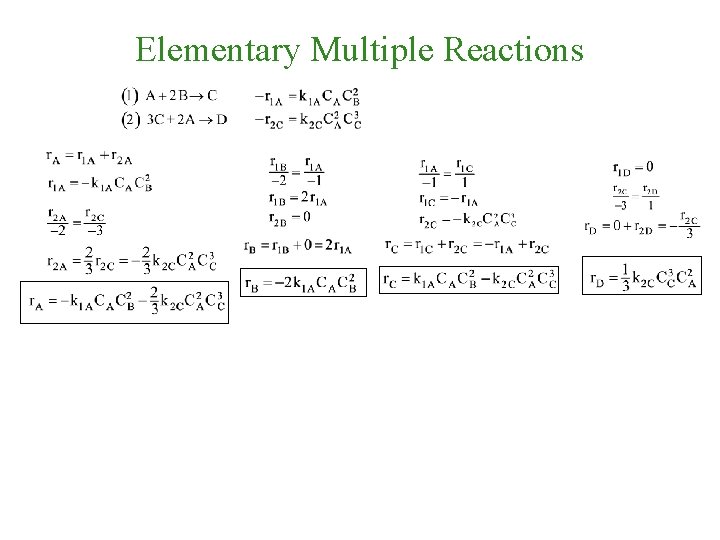
Elementary Multiple Reactions
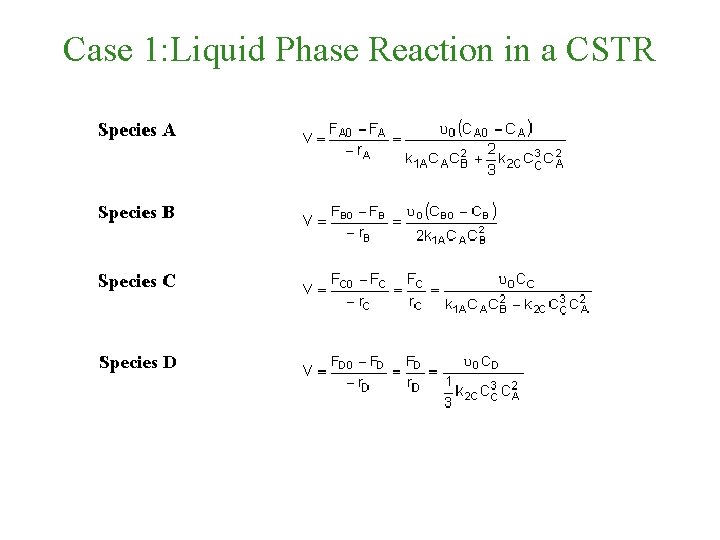
Case 1: Liquid Phase Reaction in a CSTR
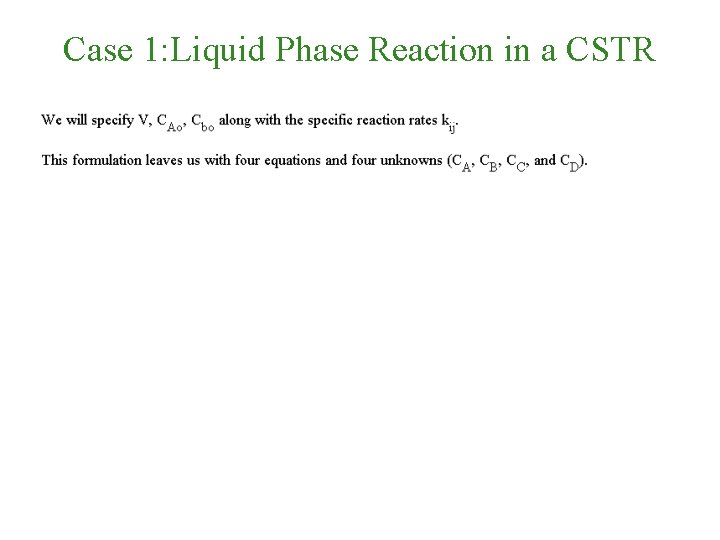
Case 1: Liquid Phase Reaction in a CSTR
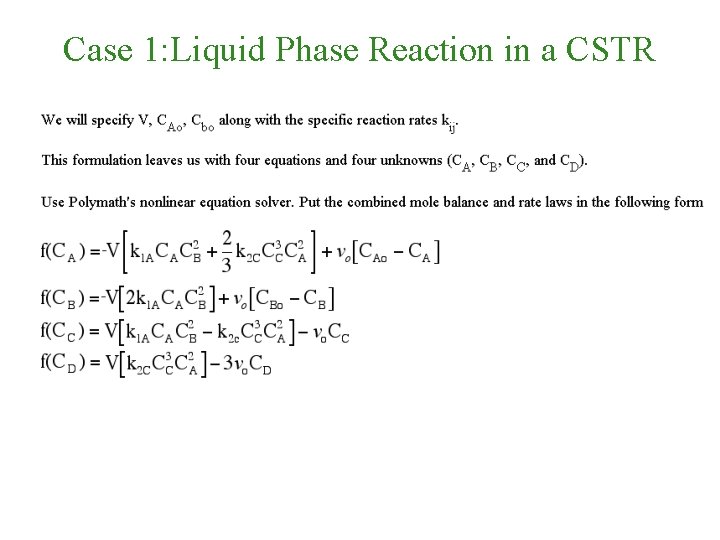
Case 1: Liquid Phase Reaction in a CSTR

Polymath Solution

Polymath Solution
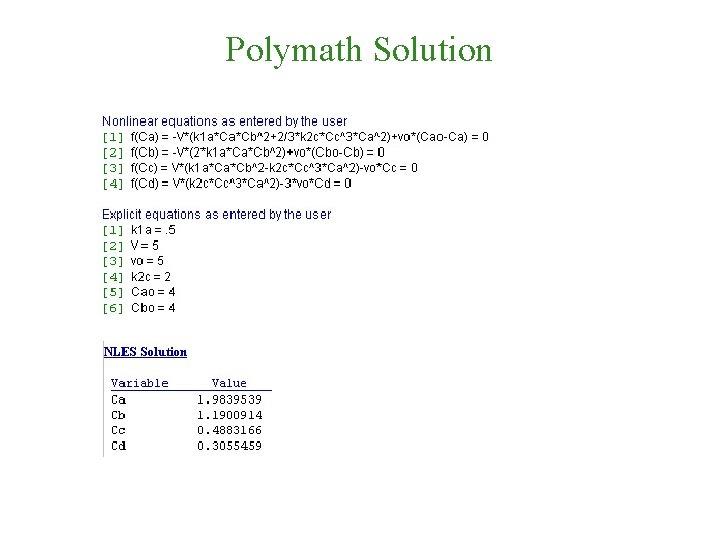
Polymath Solution
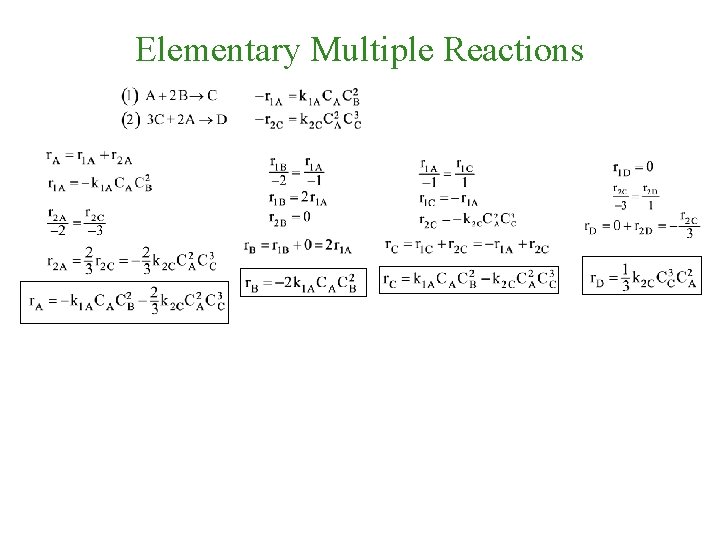
Elementary Multiple Reactions
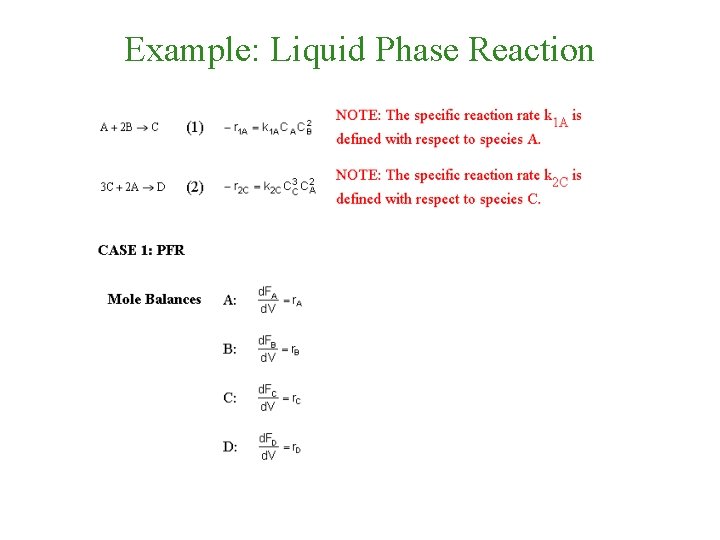
Example: Liquid Phase Reaction
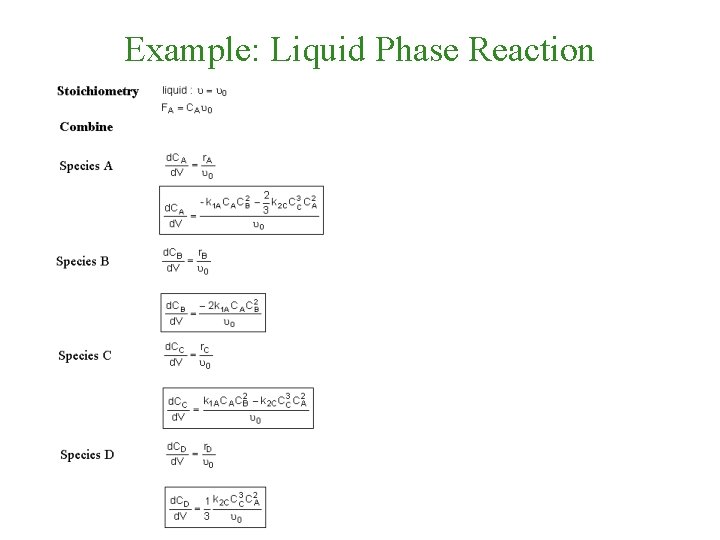
Example: Liquid Phase Reaction
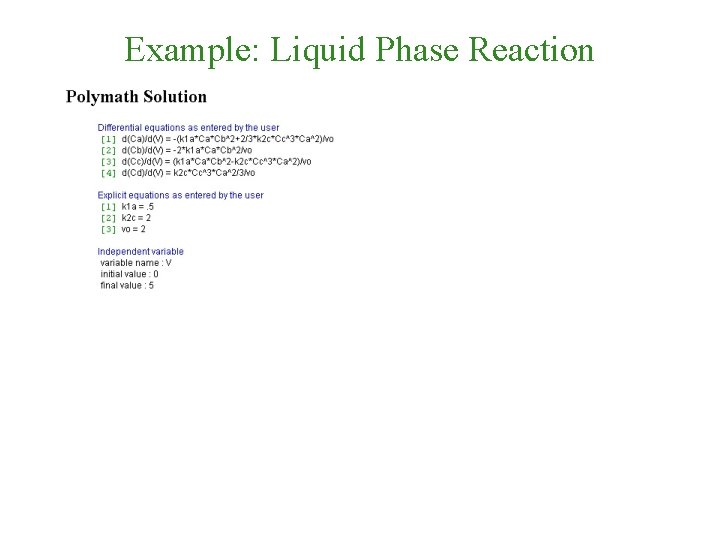
Example: Liquid Phase Reaction
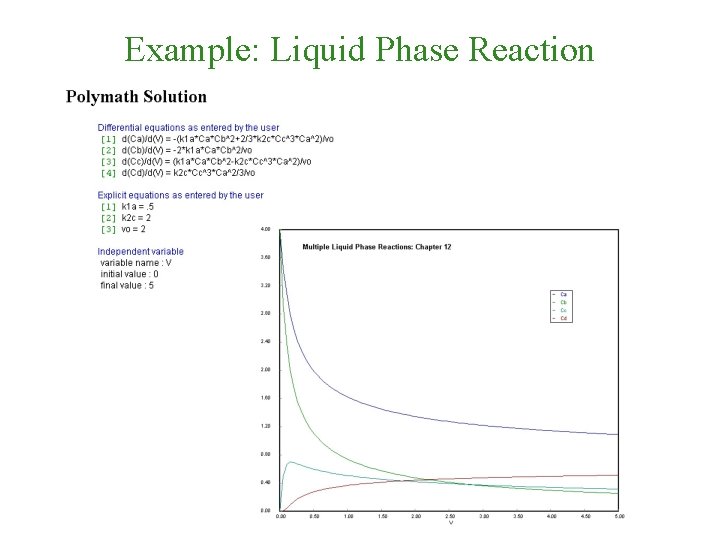
Example: Liquid Phase Reaction
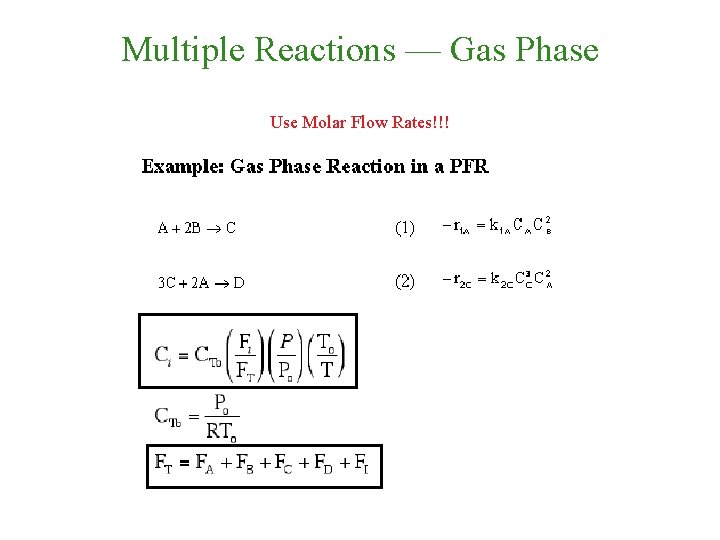
Multiple Reactions — Gas Phase Use Molar Flow Rates!!!
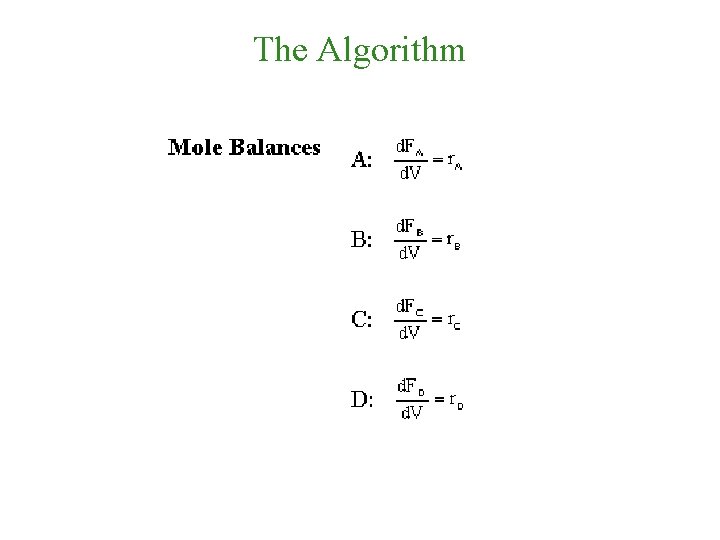
The Algorithm
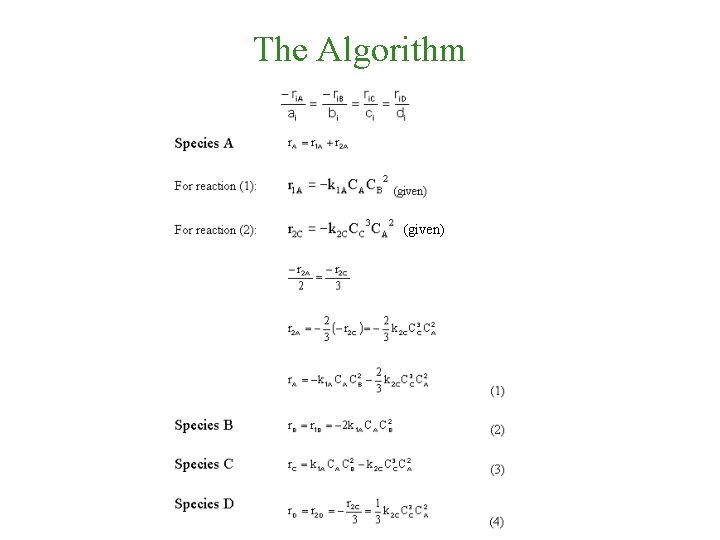
The Algorithm (given)
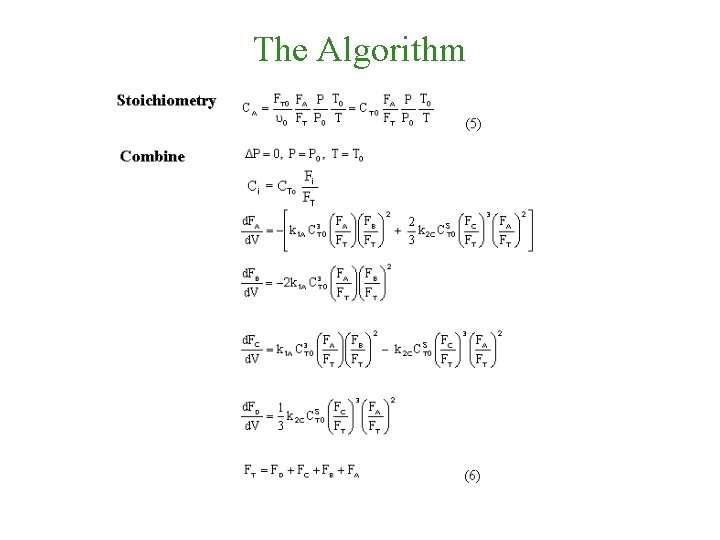
The Algorithm
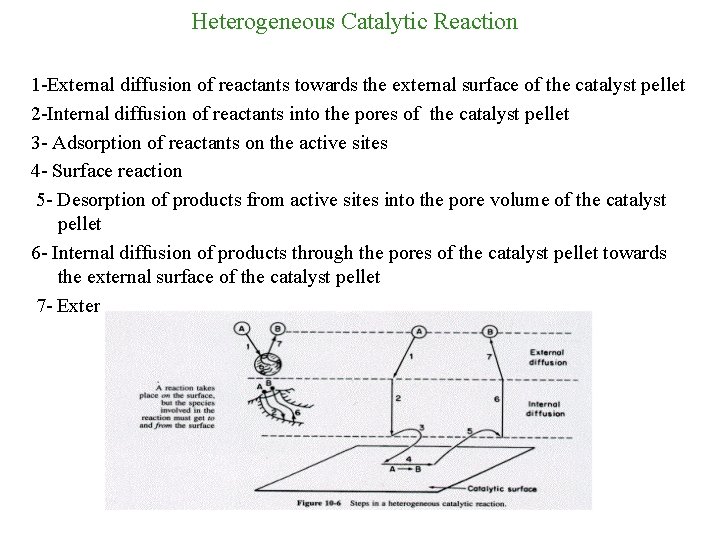
Heterogeneous Catalytic Reaction 1 -External diffusion of reactants towards the external surface of the catalyst pellet 2 -Internal diffusion of reactants into the pores of the catalyst pellet 3 - Adsorption of reactants on the active sites 4 - Surface reaction 5 - Desorption of products from active sites into the pore volume of the catalyst pellet 6 - Internal diffusion of products through the pores of the catalyst pellet towards the external surface of the catalyst pellet 7 - External diffusion of products to the bulk of the fluid

Molecular Adsorption
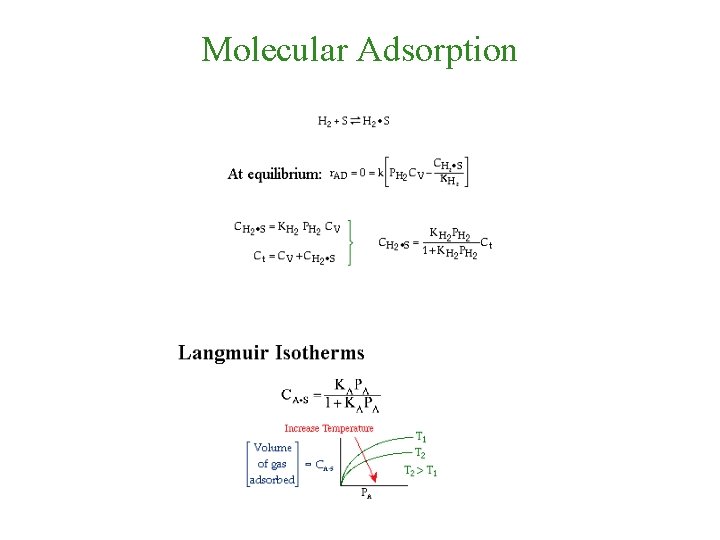
Molecular Adsorption
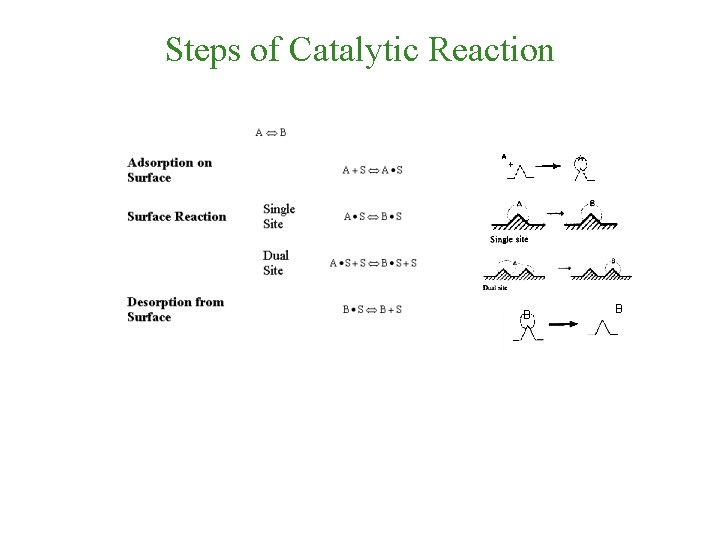
Steps of Catalytic Reaction B B
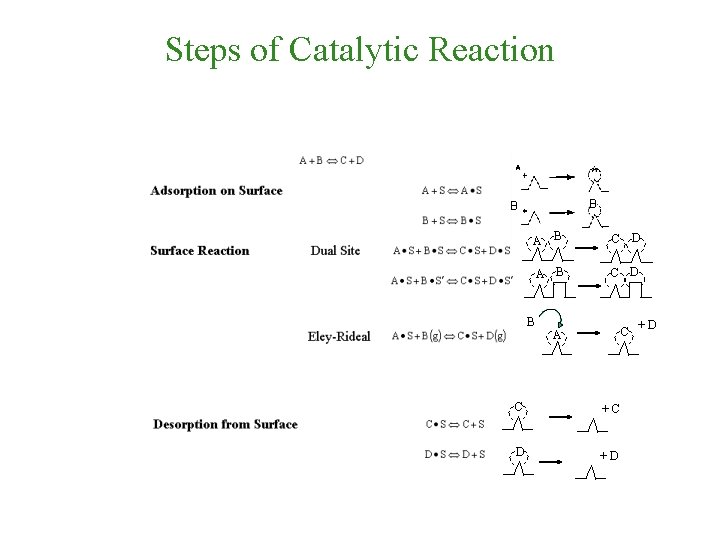
Steps of Catalytic Reaction B B B C D A B C A C +C D +D +D
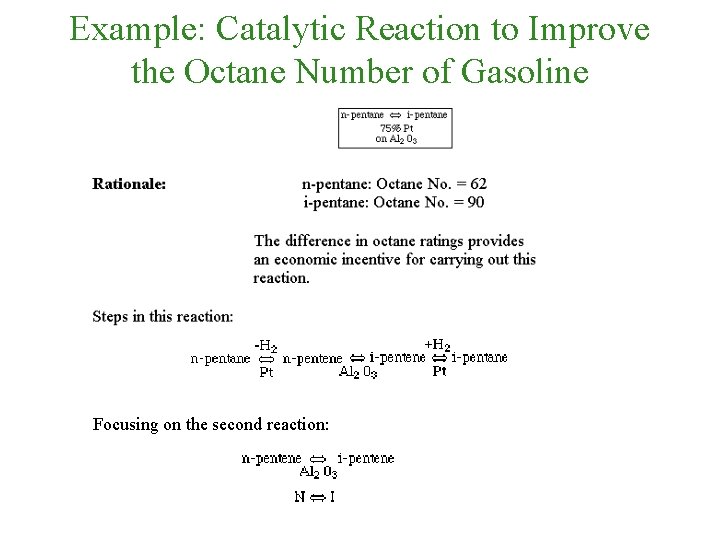
Example: Catalytic Reaction to Improve the Octane Number of Gasoline Focusing on the second reaction:
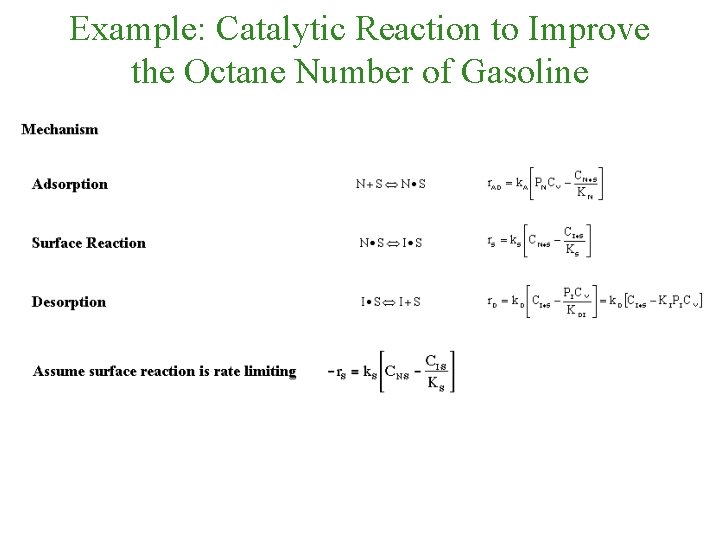
Example: Catalytic Reaction to Improve the Octane Number of Gasoline
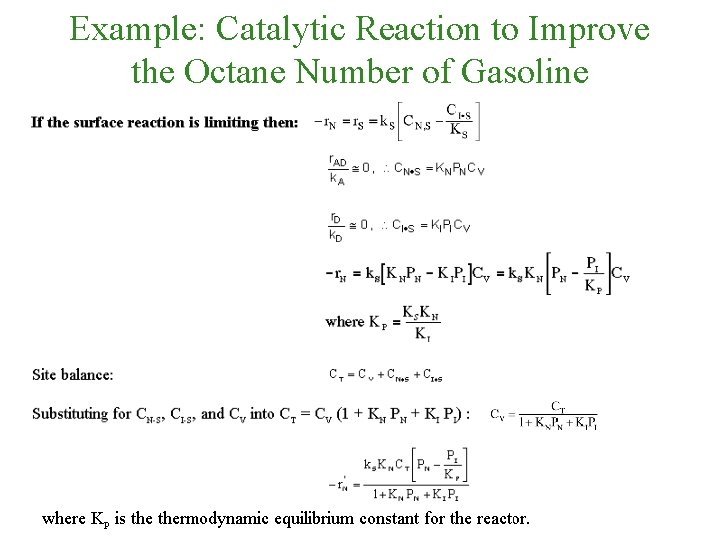
Example: Catalytic Reaction to Improve the Octane Number of Gasoline
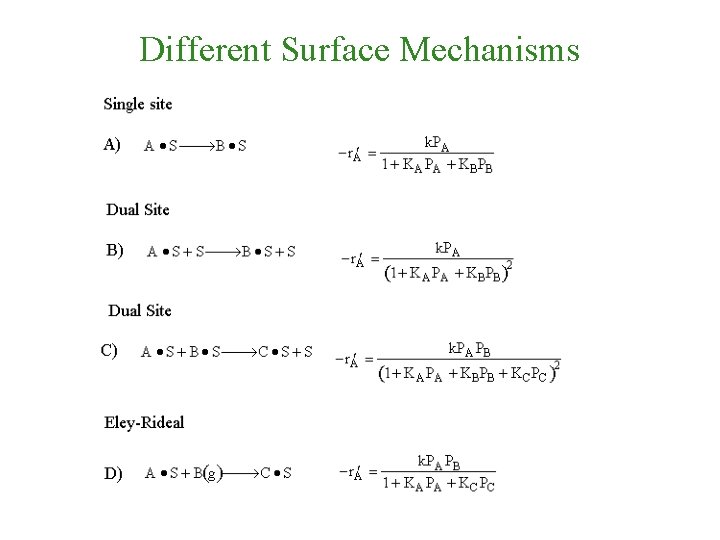
Different Surface Mechanisms
- Slides: 38