Batch Distillation In differential distillation a feed mixture
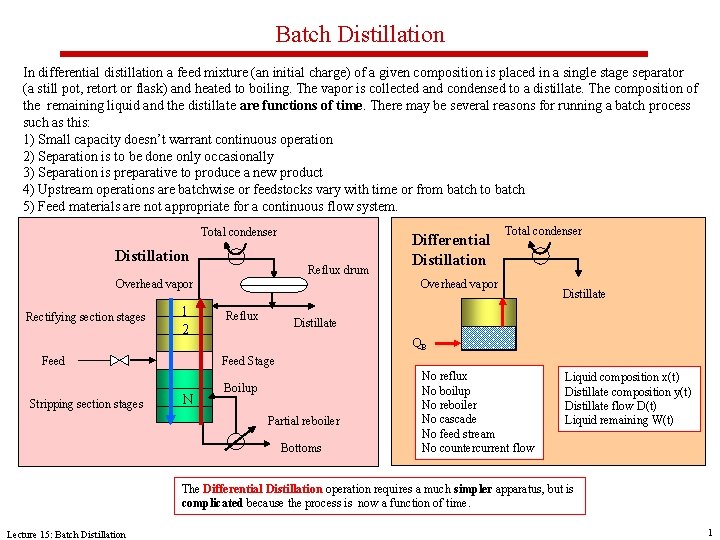
Batch Distillation In differential distillation a feed mixture (an initial charge) of a given composition is placed in a single stage separator (a still pot, retort or flask) and heated to boiling. The vapor is collected and condensed to a distillate. The composition of the remaining liquid and the distillate are functions of time. There may be several reasons for running a batch process such as this: 1) Small capacity doesn’t warrant continuous operation 2) Separation is to be done only occasionally 3) Separation is preparative to produce a new product 4) Upstream operations are batchwise or feedstocks vary with time or from batch to batch 5) Feed materials are not appropriate for a continuous flow system. Total condenser Distillation Reflux drum 1 2 Reflux Distillate QB Feed Stage Feed Stripping section stages Total condenser Overhead vapor Rectifying section stages Differential Distillation N Boilup Partial reboiler Bottoms No reflux No boilup No reboiler No cascade No feed stream No countercurrent flow Liquid composition x(t) Distillate composition y(t) Distillate flow D(t) Liquid remaining W(t) The Differential Distillation operation requires a much simpler apparatus, but is complicated because the process is now a function of time. Lecture 15: Batch Distillation 1

Batch Distillation To analyze this process we must perform component balances in the form of rates: The rate of depletion of the liquid is equal to the rate of distillate output The instantaneous rate of depletion of a component in the liquid is given by: Differential Distillation Overhead vapor Total condenser Distillate D(t), y=y. D=x. D QB Liquid left in still W(t), x=xw Change in total amount of that component in the liquid Change in composition in the liquid Change in the total amount of the liquid The instantaneous rate of the component leaving in the distillate is: Conservation of species requires that these two rates be equal to each other: Lecture 15: Batch Distillation 2

Batch Distillation Rate of depletion equals the component flow rate in Distillate Differential Distillation Overhead vapor Multiplying the above equation by dt gives: Total condenser Distillate D(t), y=y. D=x. D QB But we know that the rate of total liquid depletion is equal to the flow rate of distillate: Liquid left in still W(t), x=xw Which then gives: Rearranging to use separation of variables gives: The distillate composition and liquid composition are related through an equilibrium equation (y=kx). We can then integrate both sides: Lecture 15: Batch Distillation 3
Batch Distillation If the mixture is a binary and the relative volatility constant we can substitute the relationship: Differential Distillation Overhead vapor Total condenser Distillate D(t), y=y. D=x. D QB Into Liquid left in still W(t), x=xw and then integrate both sides to obtain: The average composition of the liquid and vapor during the process is just: The average composition of the distillate is the amount of light key vaporized divided by the amount of material vaporized. Lecture 15: Batch Distillation 4
Batch Distillation Procedure: Given an amount and composition of a feed stock, the equilibrium data for the liquid-vapor equilibrium, Rate D, and pressure: 1) Determine W using the W(x) relationship derived above for x from x 0 to xf in increments. Differential Distillation Total condenser Overhead vapor Distillate D(t), y=y. D=x. D QB Liquid left in still W(t), x=xw 2) Relate y to time by using x as a function of time and V T 3) Relate W (and thus x) to time using the rate D: t=(W 0 -W)/D 4) Determine T as a function of t, x and W by using the equilibrium data (which relates T to y and x) Lecture 15: Batch Distillation L 0 y. D(t) x (t) XB 1 5
Batch Distillation Example: A batch still is loaded with 100 kmol 50% benzene in toluene with a relative volatility = 2. 41. The boilup rate is constant at 10 kmol/hr. 1) Determine W using the W(x) relationship derived above for x from x 0 to xf in increments. Differential Distillation Overhead vapor Total condenser Distillate D(t), y=y. D=x. D QB Liquid left in still W(t), x=xw 2) Relate y to time by using x as a function of time and 3) Relate W (and thus x) to time using the rate D: t=(W 0 -W)/D 4) Determine T as a function of t, x and W by using the equilibrium data (which relates T to y and x) Lecture 15: Batch Distillation 6
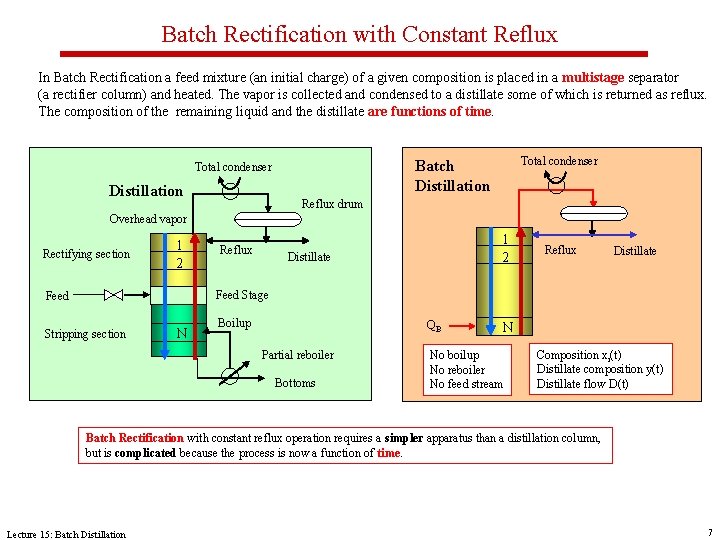
Batch Rectification with Constant Reflux In Batch Rectification a feed mixture (an initial charge) of a given composition is placed in a multistage separator (a rectifier column) and heated. The vapor is collected and condensed to a distillate some of which is returned as reflux. The composition of the remaining liquid and the distillate are functions of time. Distillation Total condenser Batch Distillation Total condenser Reflux drum Overhead vapor Rectifying section 1 2 Reflux 1 2 Distillate Reflux Distillate Feed Stage Feed Stripping section N Boilup QB Partial reboiler Bottoms N No boilup No reboiler No feed stream Composition xi(t) Distillate composition y(t) Distillate flow D(t) Batch Rectification with constant reflux operation requires a simpler apparatus than a distillation column, but is complicated because the process is now a function of time. Lecture 15: Batch Distillation 7
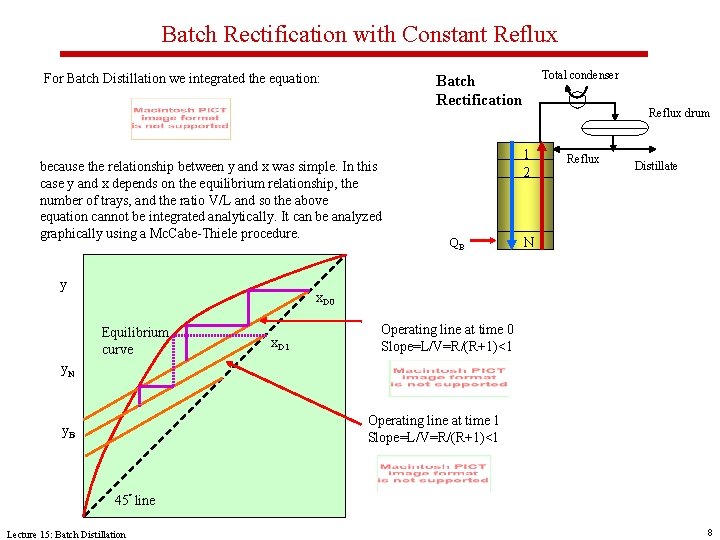
Batch Rectification with Constant Reflux For Batch Distillation we integrated the equation: because the relationship between y and x was simple. In this case y and x depends on the equilibrium relationship, the number of trays, and the ratio V/L and so the above equation cannot be integrated analytically. It can be analyzed graphically using a Mc. Cabe-Thiele procedure. y Total condenser Batch Rectification Reflux drum 1 2 QB Reflux Distillate N x. D 0 Equilibrium curve x. D 1 Operating line at time 0 Slope=L/V=R/(R+1)<1 y. N Operating line at time 1 Slope=L/V=R/(R+1)<1 y. B 45° line Lecture 15: Batch Distillation 8
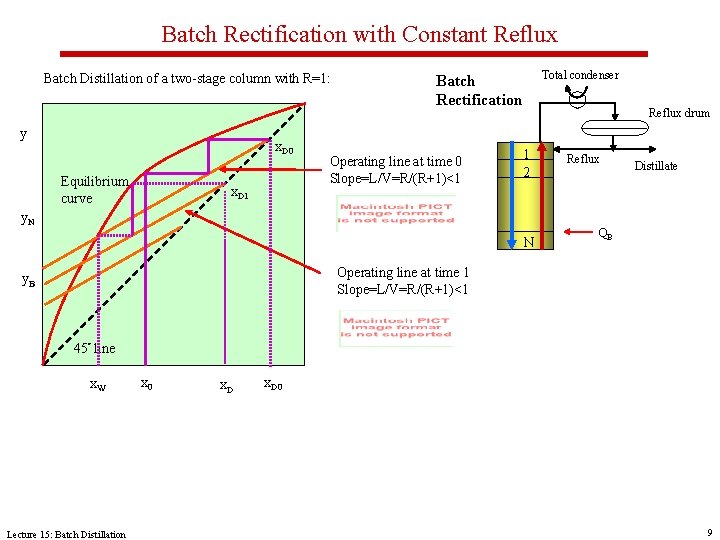
Batch Rectification with Constant Reflux Batch Distillation of a two-stage column with R=1: y x. D 0 Equilibrium curve x. D 1 Total condenser Batch Rectification Operating line at time 0 Slope=L/V=R/(R+1)<1 Reflux drum 1 2 y. N N Reflux Distillate QB Operating line at time 1 Slope=L/V=R/(R+1)<1 y. B 45° line x. W Lecture 15: Batch Distillation x 0 x. D 0 9
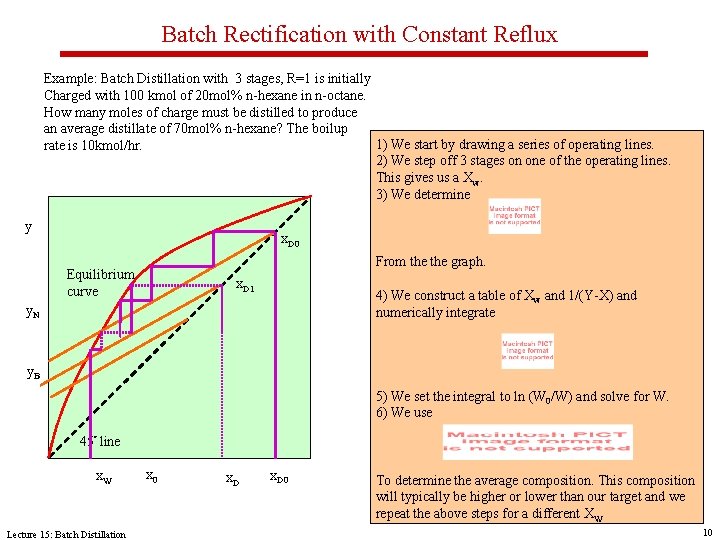
Batch Rectification with Constant Reflux Example: Batch Distillation with 3 stages, R=1 is initially Charged with 100 kmol of 20 mol% n-hexane in n-octane. How many moles of charge must be distilled to produce an average distillate of 70 mol% n-hexane? The boilup 1) We start by drawing a series of operating lines. rate is 10 kmol/hr. 2) We step off 3 stages on one of the operating lines. This gives us a Xw. 3) We determine y x. D 0 From the graph. Equilibrium curve x. D 1 4) We construct a table of Xw and 1/(Y-X) and numerically integrate y. N y. B 5) We set the integral to ln (W 0/W) and solve for W. 6) We use 45° line x. W Lecture 15: Batch Distillation x 0 x. D 0 To determine the average composition. This composition will typically be higher or lower than our target and we repeat the above steps for a different XW 10
- Slides: 10