Basics of Matter Elements All matter is made

Basics of Matter
Elements ► All matter is made of one or more elements ► Elements are substances that can’t be separated into simpler substances ► There are over 100 different elements ► Human body is mostly : carbon, hydrogen, oxygen, nitrogen, phosphorus and sulfur
Chemical Symbols ► Each element has its own symbol ► The symbol can be 1 or 2 letters ► Examples: C = Carbon Au = Gold (Aurem)
Atoms ►An atom is the smallest piece of an element ►Each element is made out of a different type of atom ►Atoms are the simplest type of particle ►An element’s properties depend on the structure of its atoms

Compounds ►Most elements are found as part of a compound ►A compound is two or more elements that have chemically combined ►Water is a compound, not an element. ►It can be separated into hydrogen and oxygen
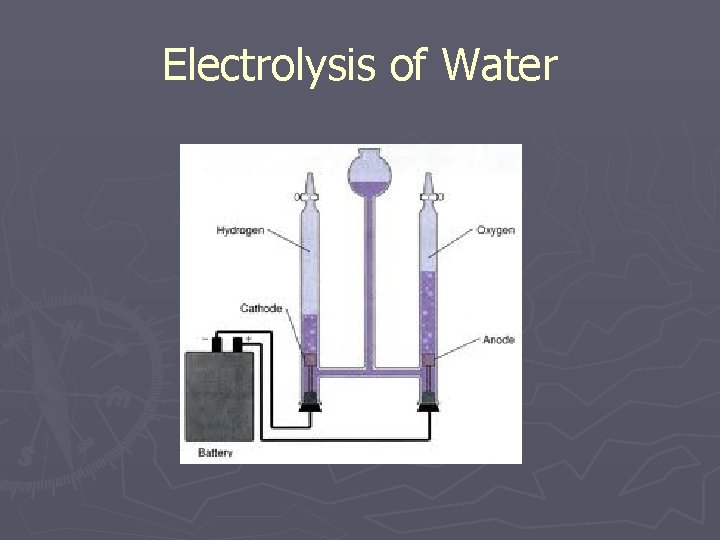
Electrolysis of Water
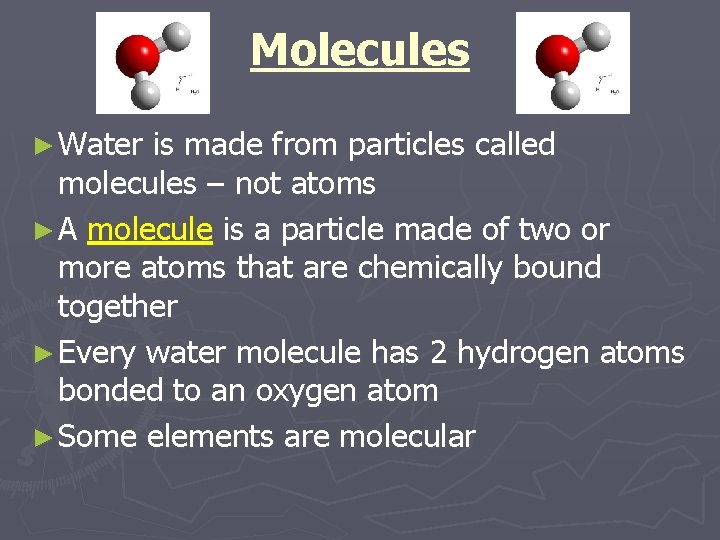
Molecules ► Water is made from particles called molecules – not atoms ► A molecule is a particle made of two or more atoms that are chemically bound together ► Every water molecule has 2 hydrogen atoms bonded to an oxygen atom ► Some elements are molecular

Atoms and molecules are the “particles” that make up all matter
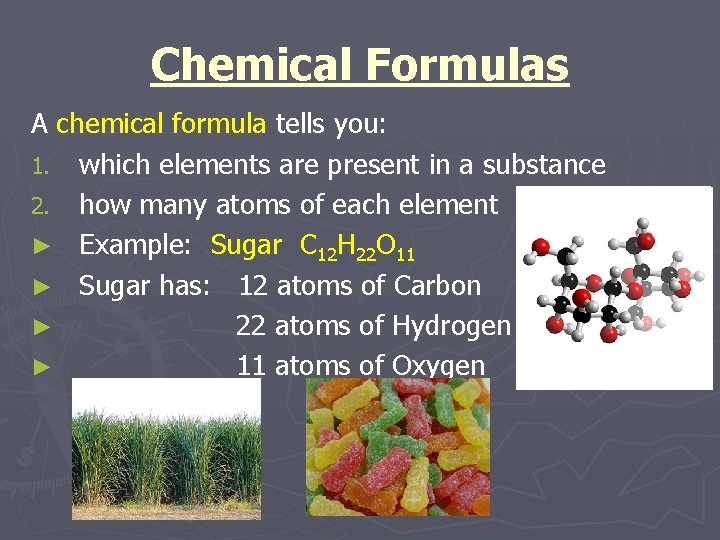
Chemical Formulas A chemical formula tells you: 1. which elements are present in a substance 2. how many atoms of each element ► Example: Sugar C 12 H 22 O 11 ► Sugar has: 12 atoms of Carbon ► 22 atoms of Hydrogen ► 11 atoms of Oxygen

Element or Compound? ► Which is the element and which is the compound? § Co ► How or CO do you know? ► How many elements are in the compounds below? § Fe 2 O 3 Rb. Br HCN H 2 SO 4

How many atoms? ► How many atoms are represented in each of the following chemical formulas? ► As 2 O 5 ► Se. O 2 ► Ag. NO 3 ► Na 2 S ► Ca(OH)2 ► (NH 4)2 SO 4
- Slides: 11