Basic Properties of the Atmosphere Heat and Temperature

Basic Properties of the Atmosphere
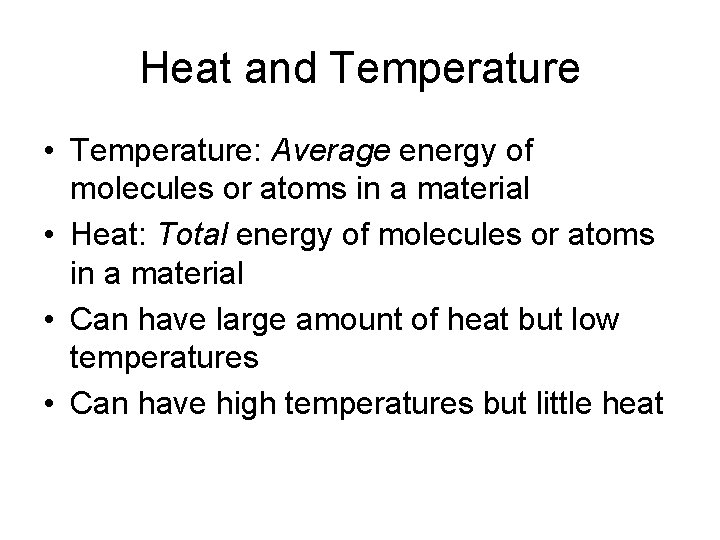
Heat and Temperature • Temperature: Average energy of molecules or atoms in a material • Heat: Total energy of molecules or atoms in a material • Can have large amount of heat but low temperatures • Can have high temperatures but little heat

Heat and Temperature • The Arctic Ocean has a large amount of heat (because of large mass) even though the temperature is low. • Air in an oven at 500 F has high temperature but little heat. • However, touch anything solid in the oven, and you’ll get burned. Same temperature, much larger amount of heat.

Heat and Temperature • The earth’s outermost atmosphere is extremely “hot” but its heat content is negligible • The surface of the moon can reach 250 F in sunlight and -200 F in shadow, but the vacuum around the Apollo astronauts contained no heat. • It takes time for things to warm up and cool off.
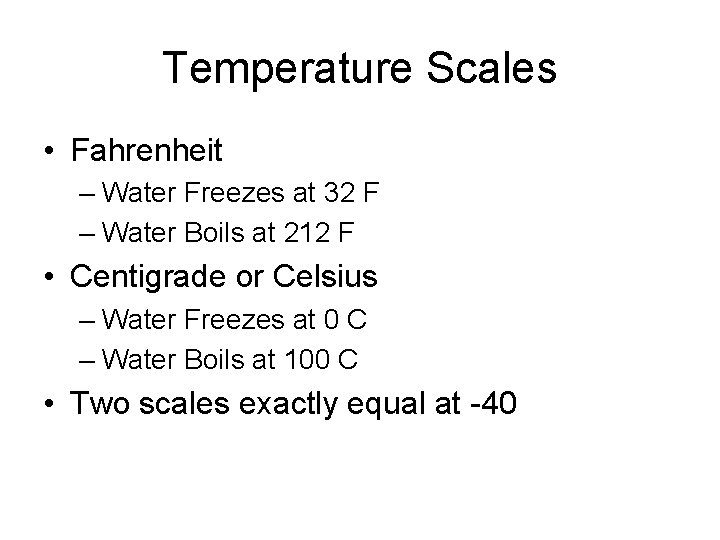
Temperature Scales • Fahrenheit – Water Freezes at 32 F – Water Boils at 212 F • Centigrade or Celsius – Water Freezes at 0 C – Water Boils at 100 C • Two scales exactly equal at -40

Converting C to F – In Your Head • Double the Centigrade • Subtract the first Digit • Add 32

Converting F to C – In Your Head • Subtract 32 • Add the first Digit • Divide by two

Absolute Temperature • Once atoms stop moving, that’s as cold as it can get • Absolute Zero = -273 C = -459 F • Kelvin scale uses Celsius degrees and starts at absolute zero • Most formulas involving temperature use the Kelvin Scale
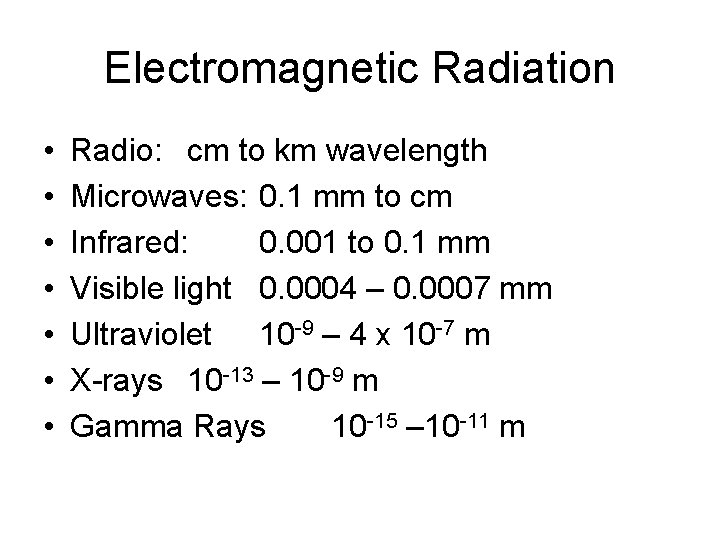
Electromagnetic Radiation • • Radio: cm to km wavelength Microwaves: 0. 1 mm to cm Infrared: 0. 001 to 0. 1 mm Visible light 0. 0004 – 0. 0007 mm Ultraviolet 10 -9 – 4 x 10 -7 m X-rays 10 -13 – 10 -9 m Gamma Rays 10 -15 – 10 -11 m
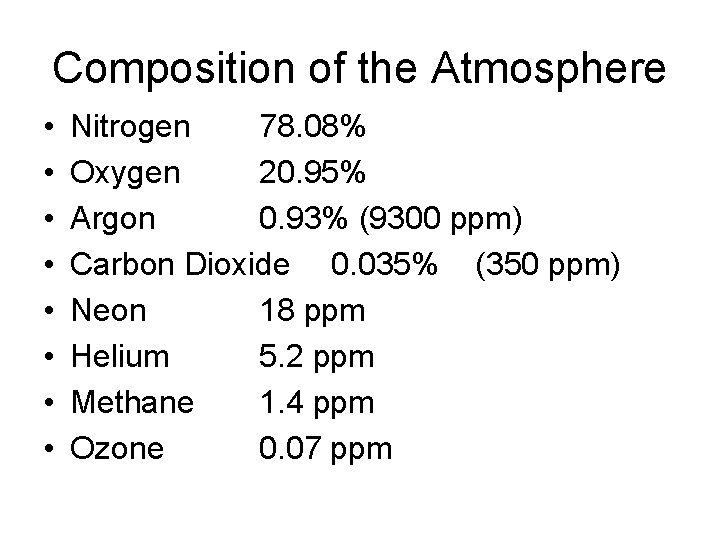
Composition of the Atmosphere • • Nitrogen 78. 08% Oxygen 20. 95% Argon 0. 93% (9300 ppm) Carbon Dioxide 0. 035% (350 ppm) Neon 18 ppm Helium 5. 2 ppm Methane 1. 4 ppm Ozone 0. 07 ppm

Other Components of the Atmosphere • • Water Droplets Ice Crystals Sulfuric Acid Aerosols Volcanic Ash Windblown Dust Sea Salt Human Pollutants

Structure of the Atmosphere • Defined by Temperature Profiles • Troposphere – Where Weather Happens • Stratosphere – Ozone Layer • Mesosphere • Thermosphere – Ionosphere

Troposphere • Heating of the Surface creates warm air at surface • Warm air rises, but air expands as it rises and cools as it expands (Adiabatic cooling) • Heating + Adiabatic Cooling = Warm air at surface, cooler air above • Buoyancy = Cool air at surface, warmer air above • Two opposing tendencies = constant turnover

Stratosphere • • Altitude 11 -50 km Temperature increases with altitude -60 C at base to 0 C at top Reason: absorption of solar energy to make ozone at upper levels (ozone layer) • Ozone (O 3) is effective at absorbing solar ultraviolet radiation

Mesosphere • • 50 – 80 km altitude Temperature decreases with altitude 0 C at base, -95 C at top Top is coldest region of atmosphere

Thermosphere • 80 km and above • Temperature increases with altitude as atoms accelerated by solar radiation • -95 C at base to 100 C at 120 km • Heat content negligible • Traces of atmosphere to 1000 km • Formerly called Ionosphere
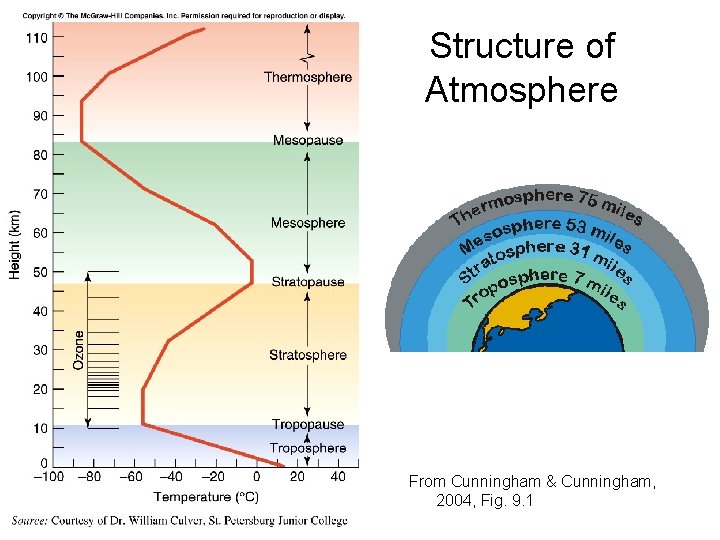
Structure of Atmosphere From Cunningham & Cunningham, 2004, Fig. 9. 1
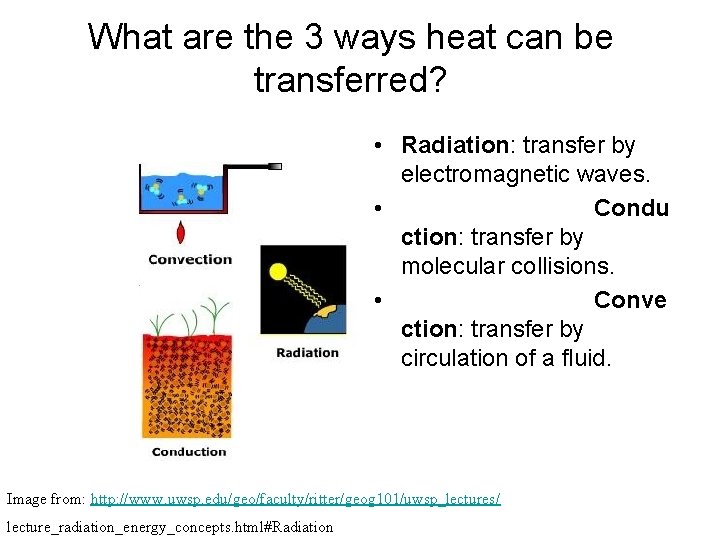
What are the 3 ways heat can be transferred? • Radiation: transfer by electromagnetic waves. • Condu ction: transfer by molecular collisions. • Conve ction: transfer by circulation of a fluid. Image from: http: //www. uwsp. edu/geo/faculty/ritter/geog 101/uwsp_lectures/ lecture_radiation_energy_concepts. html#Radiation

Why is the Mesosphere so Cold? • Stratosphere warmed because of ozone layer • Thermosphere warmed by atoms being accelerated by sunlight • Mesosphere is sandwiched between two warmer layers

Composition and Altitude • Up to about 80 km, atmospheric composition is uniform (troposphere, stratosphere, mesosphere) • This zone is called the homosphere • Above 80 km light atoms rise • This zone is sometimes called the heterosphere

Mean Free Path • Below 80 km, an atom accelerated by solar radiation will very soon hit another atom • Energy gets evenly distributed • Above 80 km atoms rarely hit other atoms • Light atoms get accelerated more and fly higher • Few atoms escape entirely

Planets and Atmospheres • At top of atmosphere, an atom behaves like any ballistic object • Velocity increases with temperature • If velocity exceeds escape velocity, atom or molecule escapes • Earth escape velocity 11 km/sec. • Moon escape velocity 2. 4 km/sec
Atmospheric Measurements • • Temperature Pressure Humidity Wind Velocity and Direction
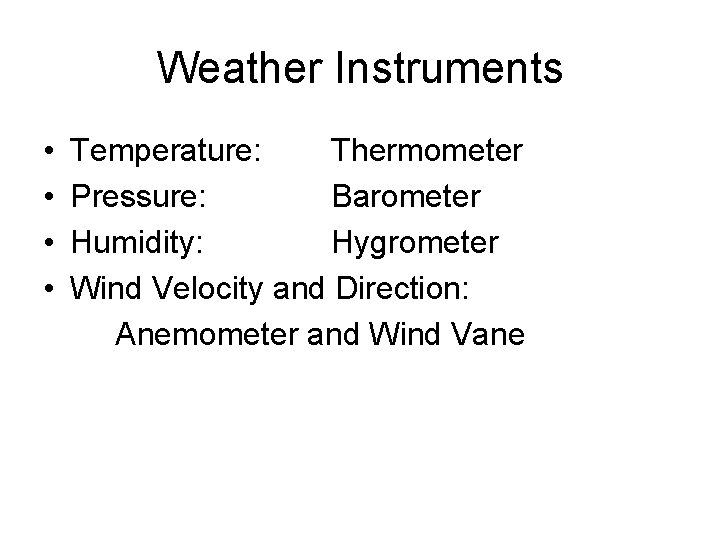
Weather Instruments • • Temperature: Thermometer Pressure: Barometer Humidity: Hygrometer Wind Velocity and Direction: Anemometer and Wind Vane
Thermometers • Fluid – Mercury – Alcohol – Use expansion of fluid • Bimetallic – Differential expansion of different metals • Electronic – Electrical resistance change with temperature

Barometers • Mercury – Air pressure will support 10 meters of water – Mercury is 13 times denser – Air pressure will support 76 cm of mercury • Aneroid – Air pressure deforms an evacuated chamber

Hygrometers • Filament – Hair expands and contracts with humidity • Sling Psychrometer – Measures cooling by evaporation – Two thermometers – Wet bulb and Dry bulb • Electrical – Chemicals change resistance as they absorb moisture

Sounding • Balloons carry radiosondes – Thermometer – Barometer – Hygrometer – Transmitter • Typically reach 30 km before balloon breaks
Radar • Detect precipitation types and amounts • Doppler radar measures velocity of winds

Satellite Studies • Visual imagery • Infrared imagery • Laser spectroscopy

Earth’s Radiation Budget • What comes in must go out • Direct Reflectance (Short Wave) – 31% • Infrared Re-emission (Long Wave) – 69%

How Heat Moves • Radiation • Conduction • Convection

Albedo = % incident energy reflected by a body • Fresh snow: 75 – 95% • Old snow: 40 – 60% • Desert: 25 – 30% • Deciduous forest, grassland: 15 – 20% • Conifer forest: 5 – 15% • Camera light meters set to 18%
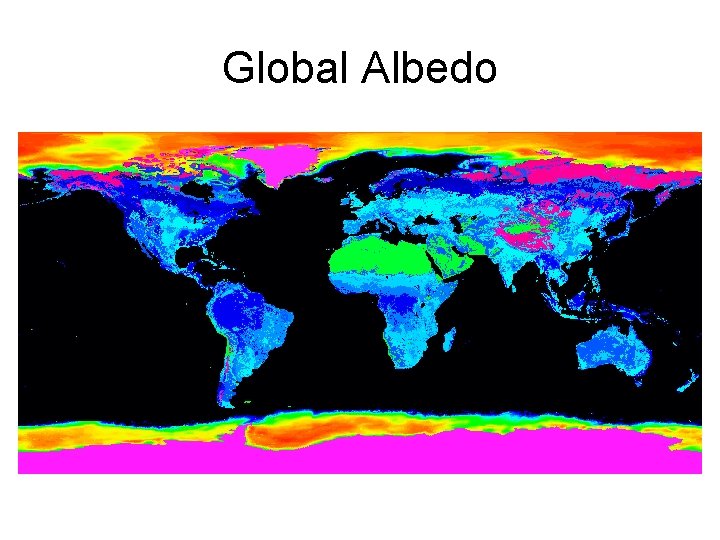
Global Albedo

Air Pressure • By lucky coincidence, earth’s atmospheric pressure is approximately neat round numbers in metric terms • 14. 7 pounds per square inch (1 kg/cm 2) • Pressure of ten meters of water • Approximately one bar or 100 k. Pa • Weather reports use millibars (mb) • One mb = pressure of one cm water

Pressure and Altitude • Average at sea level 1013 mb • 500 mb at 5 km (upper limit of human settlement) • 280 mb at 10 km • 56 mb at 20 km • 1 mb at 50 km • 0. 00056 mb at 100 km

Pressure and Altitude
- Slides: 37