Basic Principles of Measurement in Voltamperometry Electrical current
![Basic Principles of Measurement in Voltamperometry Electrical current [ampere A] flow (movement) of electric Basic Principles of Measurement in Voltamperometry Electrical current [ampere A] flow (movement) of electric](https://slidetodoc.com/presentation_image_h2/907a63ffd0a2a48539b2512507198d00/image-1.jpg)
Basic Principles of Measurement in Voltamperometry Electrical current [ampere A] flow (movement) of electric charge (charge over time) ampere - SI base unit direct current DC: constant polarity alternating current AC: periodic change of polarity electric current flow in metals: in semiconductors: in electrolytes: current direction technical: physical: in metals, semic. : in electrolytes: electrons (conceptually also positive „holes“) ions from + to – (per definition, historic) from – to + (electrons) electrons from – to + ions according to charge (to opposite polarity) cathode: electrode where reduction occurs: - in electrolytic cells, + in galvanic cells anode: electrode where oxidation occurs: + in electrolytic cells, - in galvanic cells

Redox Reactions Electrode potential for redox reaction ox + n e- red electrode potential in equilibrium in the absence of current flow Nernst equation E electrode potential [V] E° standard reduction potential [V] R gas constant (R = 8, 3145 J. mol'1. K") T absolute temperature [K] n number of transfered electrons F Faraday constant (1 F = 96485 Coulomb) a activity bei T = 298 K; Konversion ln log
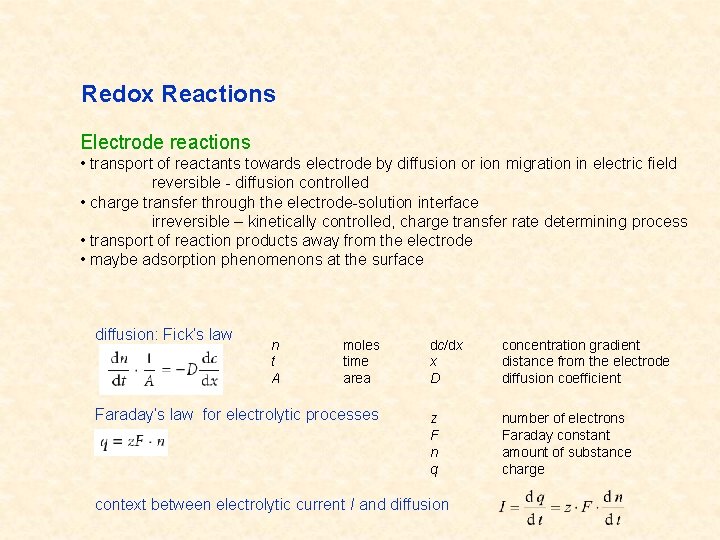
Redox Reactions Electrode reactions • transport of reactants towards electrode by diffusion or ion migration in electric field reversible - diffusion controlled • charge transfer through the electrode-solution interface irreversible – kinetically controlled, charge transfer rate determining process • transport of reaction products away from the electrode • maybe adsorption phenomenons at the surface diffusion: Fick‘s law n t A moles time area Faraday‘s law for electrolytic processes dc/dx x D concentration gradient distance from the electrode diffusion coefficient z F n q number of electrons Faraday constant amount of substance charge context between electrolytic current I and diffusion

Ion Exchange Phenomena ion exchange - one ion type is released, another is trapped by loose binding forces - usually at the surface of polyelectrolytes - some natural minerals are polyelectrolytes, among them the zeolites - more common synthetic organic resins with sites able to trap or release ions charged groups able to interact with ions of opposite charge - other sorts of phase boundary with ion exchange capability modified surfaces with charged groups interface between water and organic solvent with dissolved amphiphilic (amphipathic) substances accumulated at the interface cation exchangers possess negatively charged groups, may exchange cations strongly acidic – negatively charged over the whole p. H-range (e. g. -SO 3 -) weakly acidic – charged only in higher p. H-range (e. g. –COOH) anion exchangers possess positively charged groups, may exchange anions strongly basic – positively charged over the whole p. H-range (e. g. -NR 3+) weakly basic – charged only in lower p. H-range (e. g. –NH 2)
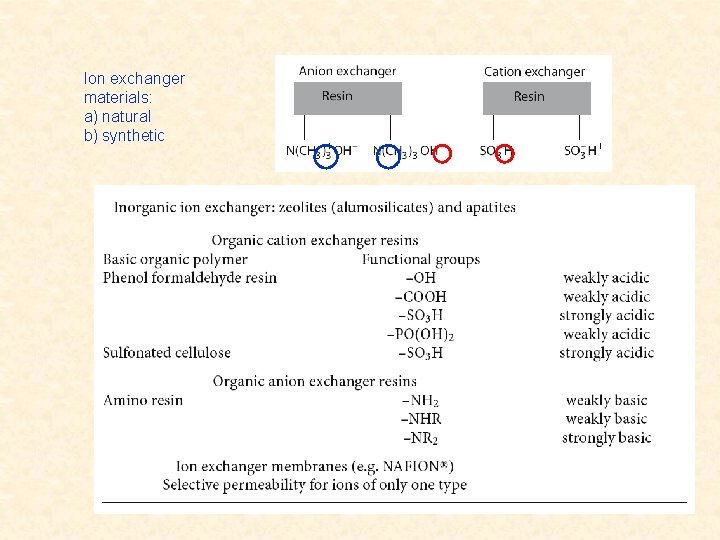
Ion exchanger materials: a) natural b) synthetic
Enzyme Reaction Enzymes are biocatalysts with an extremely high selectivity protein molecules with a molecular mass between 104 to 105 Da work under mild conditions i. e. at room temperature or slightly above usually at near-neutral p. H Biosensors with enzymes generally contain a layer of enzyme molecules immobilized at the sensor surface able to catalyse just one reaction with definite biologically active substance most important feature of enzyme molecules specific three-dimensional configuration with a molecular cavity including an active site suitable to interact with a special substrate molecule here reaction of the substrate during formation of product takes place enzyme molecule recognizes the substrate sterically by following the lock-and-key principle active site makes up only a small part of the overall molecular volume its primary function is to stabilize the activated complex molecular transition state between enzyme and substrate
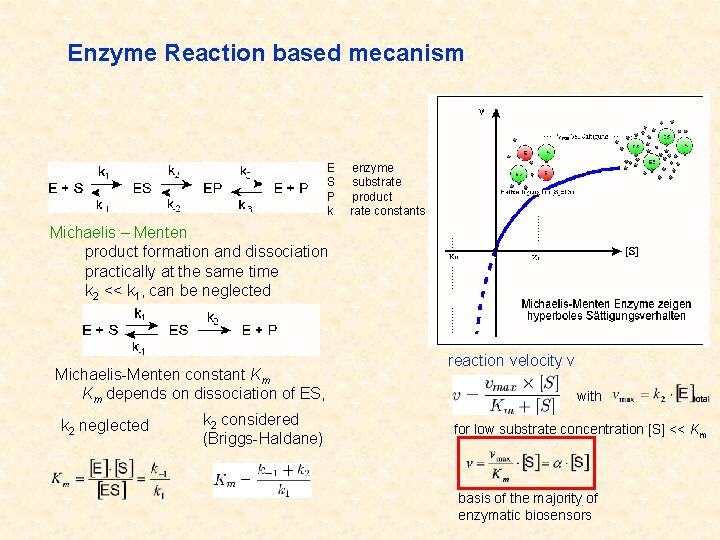
Enzyme Reaction based mecanism E S P k enzyme substrate product rate constants Michaelis – Menten product formation and dissociation practically at the same time k 2 << k 1, can be neglected Michaelis-Menten constant Km Km depends on dissociation of ES, k 2 neglected k 2 considered (Briggs-Haldane) reaction velocity v with for low substrate concentration [S] << Km basis of the majority of enzymatic biosensors
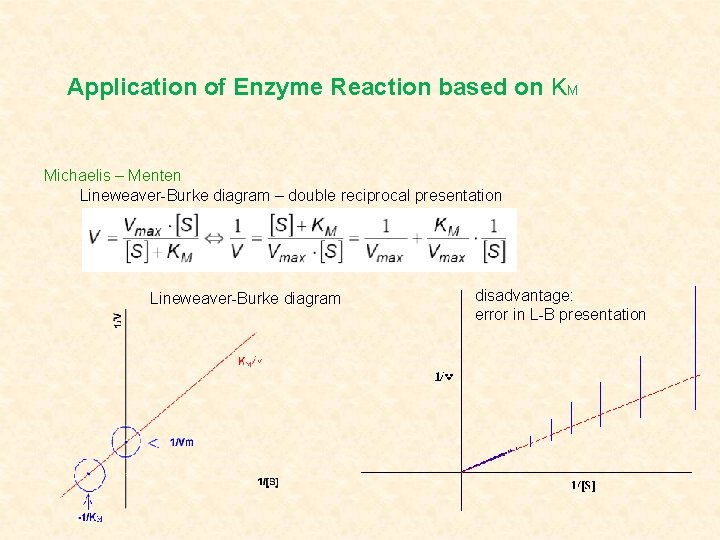
Application of Enzyme Reaction based on KM Michaelis – Menten Lineweaver-Burke diagram – double reciprocal presentation Lineweaver-Burke diagram disadvantage: error in L-B presentation
- Slides: 8