Basic Principles of GMP Quality Management Section 1

Basic Principles of GMP Quality Management Section 1 and 2 Module 2 | Slide 1 of 27 2012

Quality Management Objectives l To understand key issues in quality assurance/good manufacturing practices/quality control. l To understand specific requirements on quality management and quality assurance including: äOrganization äProcedures, processes and resources. l To develop actions to resolve your current problems. Module 2 | Slide 2 of 27 2012
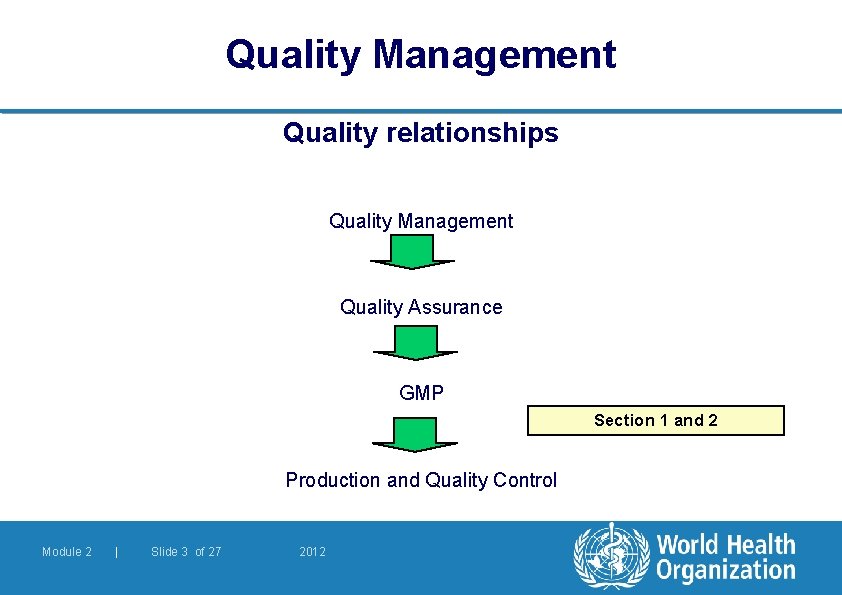
Quality Management Quality relationships Quality Management Quality Assurance GMP Section 1 and 2 Production and Quality Control Module 2 | Slide 3 of 27 2012

Quality Management Philosophy and essential elements l What is Quality Management? ä The aspect of management function that determines and implements the “quality policy” ä The overall intention and direction regarding quality, as formally expressed and authorized by top management Module 2 | Slide 4 of 27 2012

Quality Management l The basic elements are: ä An appropriate infrastructure or “quality system” encompassing the organization structure, procedures, processes and resources ä The systematic actions necessary to ensure adequate confidence that a product (or service) will satisfy given requirements for “Quality” The totality of these actions is termed “Quality Assurance” Module 2 | Slide 5 of 27 2012

Quality Management l Quality assurance is a management tool l In contractual situations, it also serves to generate confidence in a supplier l QA, GMP and Quality Control are interrelated aspects of Quality Management ä They are described on the following slides in order to emphasize their relationship and their fundamental importance to the production and control of pharmaceutical products Module 2 | Slide 6 of 27 2012

Quality Management Principles of Quality Assurance (QA) l Wide-ranging concept ä covers all matters that individually or collectively influence the quality of a product l Totality of the arrangements ä to ensure that the drug is of the right quality for the intended use 1. 1 l Quality Assurance incorporates GMP ä also product design and development Module 2 | Slide 7 of 27 2012

Quality Management QA System should ensure that: l Products are designed and developed in accordance with GLP, GCP, and GMP l Production and control operations are clearly specified in SOPs l Managerial responsibilities are clearly specified in job descriptions 1. 2 l Systems ensure that the correct starting and packaging materials are used Module 2 | Slide 8 of 27 2012

Quality Management QA System should ensure: l Starting materials, intermediate products, bulk products are controlled l In-process controls, calibrations, and validations are carried out l Finished products are correctly processed and checked l Products are not sold or supplied before release by authorized 1. 2 persons l Systems ensure that products are appropriately stored and distributed Module 2 | Slide 9 of 27 2012

Quality Management QA System should ensure: l Self-inspection and/or quality audits are done regularly l Deviations are reported, investigated and recorded l Changes are controlled l Systems are followed to verify the consistency of processes and ensuring continuous improvement l Quality Risk Management is implemented Module 2 | Slide 10 of 27 2012 1. 2

Quality Management Quality Assurance l Products must: – safety, quality and efficacy requirements - fit for their intended use – comply with the requirements of the marketing authorization l Senior management is responsible - and all staff must be committed to achieve this. l Relies on a comprehensively designed, documented, correctly implemented system of QA incorporating GMP and QC. l Relies on competent personnel, suitable and sufficient premises, equipment and facilities. 1. 3 Module 2 | Slide 11 of 27 2012

Quality Management Quality Assurance l Manufacturers should manage quality risks. Quality Risk Management (QRM) is a systematic process for: – assessment, control, communication and review of risks to the quality of the medicinal product. l QRM: – can be applied both proactively and retrospectively – Should be based on scientific knowledge and experience with the process – Should be linked to the protection of the patient 1. 4 – 1. 5 Module 2 | Slide 12 of 27 2012
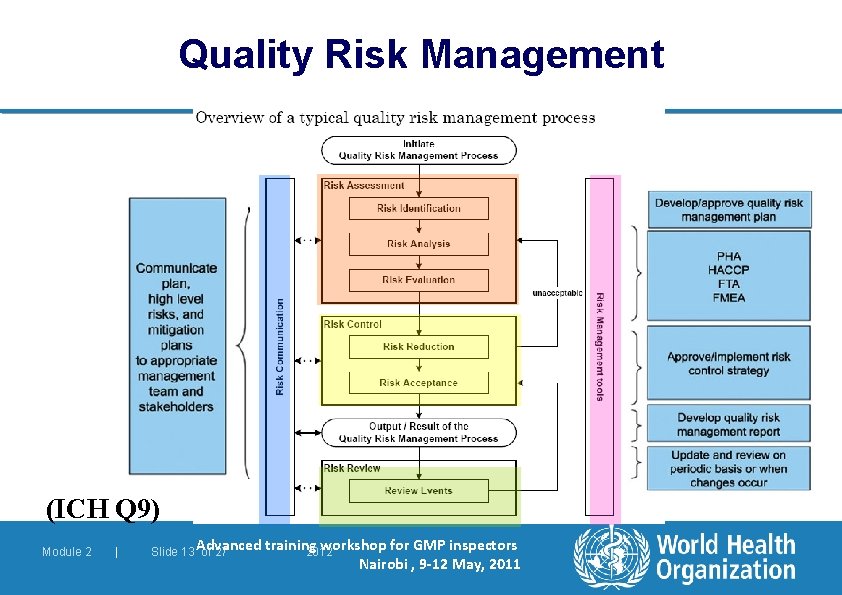
Quality Risk Management (ICH Q 9) Module 2 | Slide 13 Advanced of 27 training workshop for GMP inspectors 2012 Nairobi , 9 -12 May, 2011

Quality Management Quality Assurance l Quality Risk Management follows a cycle of assessment, control, communication and review. l An appropriate tool should be used in risk assessment, such as: – Fault Tree Analysis (FTA) – Hazard and Operational Studies (HAZOP) – Failure Mode and Effect Analysis (FMEA) – Hazard Analysis and Criticality Analysis (HACCP) – Failure Mode, Effect, and Criticality Analysis (FMECA) 1. 3 Module 2 | Slide 14 of 27 2012

Quality Management Failure Mode Effect Analysis • Breakdown in manageable steps • Process and product understanding needed • Evaluate failure mode and effect on outcome • Eliminate, contain, reduce, control • (Identify mode, cause, effect) Module 2 | Slide 15 of 27 2012

Quality Management Quality Assurance Product quality review (PQR) l Regular, periodic or rolling quality reviews of all medicinal products l Normally annually l Objective: – Verifying the consistency of the existing process – appropriateness of current specifications for both starting materials and finished product 1. 6 – highlight any trends – identify product and process improvements. Module 2 | Slide 16 of 27 2012

Quality Management Quality Assurance l PQR should include at least a review of: – starting materials and packaging materials (especially from new sources) – critical in-process controls and finished product results – all batch failures and their investigation – deviations or non-conformances (and investigations and CAPAs) – all changes made to the processes or analytical methods – dossier variations submitted, granted or refused – results of the stability monitoring programme and any adverse 1. 6 trends Module 2 | Slide 17 of 27 2012

Quality Management Quality Assurance l PQR should include (cont). : – quality-related returns, complaints and recalls and the investigations – adequacy of previous corrective actions on product process or equipment – Post marketing commitments – qualification status of relevant equipment and utilities – technical agreements 1. 6 Module 2 | Slide 18 of 27 2012

Quality Management Quality Assurance l Results should be reviewed- assessment should be made whether CAPA or revalidation should be undertaken l CAPA completed in a timely and effective manner – verified l Product types can be grouped l Agreements in case of contracted parties l PQR in a timely manner and verified for accuracy 1. 6 Module 2 | Slide 19 of 27 2012

Quality Management Quality Assurance When inspecting PQR, also verify: l Correctness of data transferred l Trending of results l Calculations such as process capability index (Cp. K) - where appropriate l Accuracy in terms of APIs reflected, approved suppliers used, number of batches, variations, changes, complaints etc. l CAPAs and conclusion Module 2 | Slide 20 of 27 2012

Quality Management Good Manufacturing Practices (GMP) l That part of QA that ensures that products are consistently produced and controlled ä Quality standards ä Marketing authorization l Aim: Diminishing risks that cannot be controlled by testing of product ä Contamination and cross-contamination 2. 1 ä Mix-ups (confusion) Module 2 | Slide 21 of 27 2012

Quality Management Basic Requirements for GMP – I l Clearly defined and systematically reviewed processes l Qualification and validation is performed l Appropriate resources are provided: ä Qualified and trained personnel ä Premises, space, equipment and services ä Materials, containers, labels ä Procedures, storage, transport ä Laboratories and in-process control Module 2 | Slide 22 of 27 2012 2. 1 a - c

Quality Management Basic Requirements for GMP – I l Clear, written instructions and procedures l Trained operators l Records of actions, deviations and investigations l Records for manufacture and distribution l Proper storage and distribution l Systems for complaints and recalls Module 2 | Slide 23 of 27 2012 2. 1 d - j

Quality Management Group session – I l How many GMP deficiencies can you find in the photographs in the handout? Module 2 | Slide 24 of 27 2012

Quality Management Group session II l Imagine you are inspecting a pharmaceutical company for compliance with GMP l Consider the situations in the next slides which may have impact on a company’s quality management programme l Describe the action to be taken in each case Module 2 | Slide 25 of 27 2012

Quality Management Issues – I l Quality Management manual not established in writing l Limited human resources l Lack of qualified people l Processes not properly validated l Poor SOPs or standard batch documentation l More consideration to cost than quality l Family members in key positions of authority Module 2 | Slide 26 of 27 2012

Quality Management Issues – II l Substandard materials deliberately purchased l Technical staff not involved in purchasing l Inability to re-export substandard materials l Owner insists on selling rejects l Corruption l No commitment to training Module 2 | Slide 27 of 27 2012
- Slides: 27