Basic Principles of GMP Quality Management Part One

Basic Principles of GMP Quality Management Part One Module 2 Slide 1 of 26 WHO - EDM

Quality Management Objectives l To understand key issues in quality assurance/quality control. l To understand specific requirements on organization, procedures, processes and resources. l To develop actions to resolve your current problems. Module 2 Slide 2 of 26 WHO - EDM

Quality Management l Determines and implements the “quality policy” l The basic elements are: ä an appropriate infrastructure or “quality system” encompassing the Procedures, Processes, and Resources ä the systematic actions necessary to ensure adequate confidence that a product (or service) will satisfy given requirements for “Quality” The totality of these actions is termed “Quality Assurance” Part One Module 2 Slide 3 of 26 WHO - EDM

Quality Management l Terminology may differ ä “Quality System” is said to be rarely used in drug manufacturing l The concepts of QA, GMP and Quality Control are interrelated aspects of Quality Management. ä They are described on the following slides in order to emphasize their relationship and their fundamental importance to the production and control of pharmaceutical products Part One Module 2 Slide 4 of 26 WHO - EDM

Quality Management Principles of Quality Assurance l Wide-ranging concept ä covers all matters that individually or collectively influence the quality of a product l Totality of the arrangements ä to ensure that the drug is of the right quality for the intended use l Quality Assurance incorporates GMP ä and also product design and development which is Part One 1. 1 outside the scope of this module Module 2 Slide 5 of 26 WHO - EDM

Quality Management Requirements for QA Systems – I 1. Ensure products are developed correctly 2. Identify managerial responsibilities 3. Provide SOPs for production and control 4. Organize supply and use of correct starting materials 5. Define controls for all stages of manufacture and packaging Part One 1. 2 a-j Module 2 Slide 6 of 26 WHO - EDM

Quality Management Requirements for QA Systems – II 6. Ensure finished product correctly processed and checked before release 7. Ensure products are released after review by authorized person 8. Provide storage and distribution 9. Organize self-inspection Part One 1. 2 a-j Module 2 Slide 7 of 26 WHO - EDM

Quality Management GMP l Ensure that products are consistently produced and controlled l Diminishes risks that cannot be controlled by testing of product ä Cross-contamination ä Mix-ups Part One 2. 1 a-j Module 2 Slide 8 of 26 WHO - EDM

Quality Management Basic Requirements for GMP – I 1. Clearly defined and systematically reviewed processes 2. Critical steps validated 3. Appropriate resources: personnel, buildings, equipment, materials 4. Clearly written procedures 5. Trained operators Part One 2. 1 a-j Module 2 Slide 9 of 26 WHO - EDM

Quality Management Basic Requirements for GMP – II 6. Complete records, failure investigations 7. Proper storage and distribution 8. Recall system 9. Complaint handling Part One 2. 1 a-j Module 2 Slide 10 of 26 WHO - EDM

Quality Management Group session - I l Module 2 How many GMP deficiencies can you find in the photographs in the handout? Slide 11 of 26 WHO - EDM
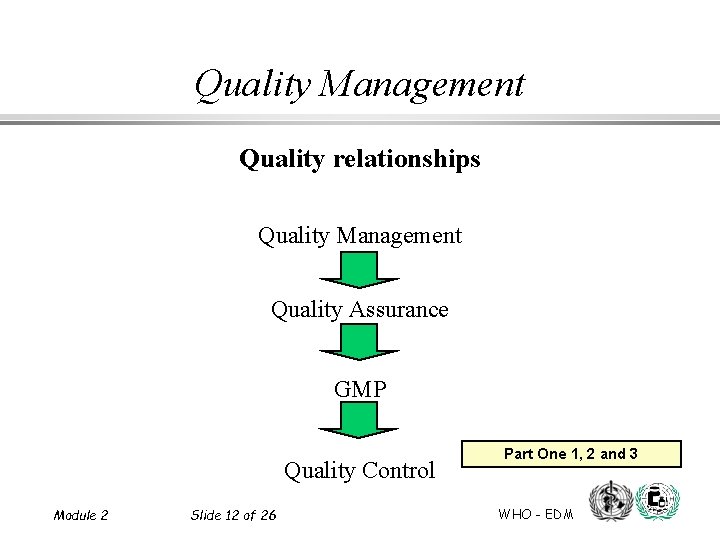
Quality Management Quality relationships Quality Management Quality Assurance GMP Quality Control Module 2 Slide 12 of 26 Part One 1, 2 and 3 WHO - EDM

Quality Management Quality Control (QC) l Module 2 QC is part of GMP Slide 13 of 26 WHO - EDM

Quality Management Quality Control (QC) Department l Each holder of a manufacturing authorization should have a QC Department l Independence from production and other departments is considered to be fundamental l Under the authority of an appropriately qualified and experienced person with one or several control laboratories at his or her disposal. Part One 3. 2 Module 2 Slide 14 of 26 WHO - EDM

Quality Management Basic Requirements for Quality Control Resources l Adequate facilities l Trained personnel l Approved procedures Part One 3. 2 Module 2 Slide 15 of 26 WHO - EDM

Quality Management Basic Requirements for Quality Control Tasks l Sampling l Inspecting l Testing l Monitoring l Releasing/rejecting Part One 3. 2 Module 2 Slide 16 of 26 WHO - EDM

Quality Management Basic Requirements for Quality Control - I Objects l Starting materials l Packaging materials l Intermediates l Bulk products l Finished products l Environmental conditions Module 2 Slide 17 of 26 Part One 3. 2 WHO - EDM

Quality Management Basic Requirements for Quality Control – II 1. Sampling approved by QC department 2. Validated test methods 3. Records 4. Review and evaluation of production documentation 5. Failure investigations for all deviations 6. Ingredients comply with the marketing authorization Part One 3. 2 b – e Module 2 Slide 18 of 26 WHO - EDM

Quality Management Basic Requirements for Quality Control – III 7. Ingredients are of the required purity 8. Proper containers 9. Correct labelling 10. Release of batches by the authorized person 11. Retained samples of starting materials and products Part One 3. 2 e – h Module 2 Slide 19 of 26 WHO - EDM

Quality Management Other Duties of the Quality Control Department 1. Establish QC procedures 2. Reference standards 3. Correct labelling 4. Stability testing 5. Complaint investigations 6. Environmental monitoring Part One 3. 3 Module 2 Slide 20 of 26 WHO - EDM

Quality Management Assessment of Finished Products Should embrace all relevant factors. For example: l production conditions l in-process test results l manufacturing documentation l compliance with finished product specification l examination of the finished pack Part One 3. 4 Module 2 Slide 21 of 26 WHO - EDM

Quality Management QC Access l QC Personnel MUST have access to production areas for sampling and investigation l As appropriate! Part One 3. 5 Module 2 Slide 22 of 26 WHO - EDM

Quality Management Quality Control - summary l QC is part of GMP - refer to the handout ä sampling ä specifications ä testing ä release procedures ä recalls and complaints ä decision-making in all quality matters Module 2 Slide 23 of 26 ä authorization ä definition of product quality ä laboratory operations ä release decisions ä investigation and reporting Part One 3. 1, 3. 2 WHO - EDM

Quality Management Group session II l Imagine you are inspecting a pharmaceutical company for compliance with GMP l Consider the situations in the next slides which may impact on a company’s quality management programme. l Describe the action to be taken in each case Module 2 Slide 24 of 26 WHO - EDM

Quality Management Issues – I l Quality Management manual not established in writing l Limited human resources l Lack of qualified people l Processes not properly validated l Poor SOPs or standard batch documentation l More consideration to cost than quality l Family members in key positions of authority Module 2 Slide 25 of 26 WHO - EDM

Quality Management Issues – II l Substandard materials deliberately purchased l Technical staff not involved in purchasing l Inability to re-export substandard materials l Owner insists on selling rejects l Corruption l No commitment to training Module 2 Slide 26 of 26 WHO - EDM
- Slides: 26