Basic Principles of GMP Documentation Part 1 15

Basic Principles of GMP Documentation Part 1 15 Module 12 – part 1 January 2006 | Slide 1 of 20 STOP

Documentation Objectives 1. To review general requirements for documents 2. To review specific requirements for each document 3. To consider current issues applicable to your countries Module 12 – part 1 January 2006 | Slide 2 of 20 STOP

Documentation General Principles – I l Good documentation is an essential part of the QA system l Should exist for all aspects of GMP l Purpose of documentation ä Defines specifications and procedures for all materials and methods of manufacture and control ä Ensures all personnel know what to do and when to do it ä Ensure that authorized persons have all information necessary for release of product 15. 1 Module 12 – part 1 January 2006 | Slide 3 of 20 STOP

Documentation General Principles – I l Purpose of documentation (cont. ) ä Ensures documented evidence, traceability, provide records and audit trail for investigation ä Ensures availability of data for validation, review and statistical analysis l Design and use ä Depends upon manufacturer ä Some documents combined into one, sometimes separate 15. 1 Module 12 – part 1 January 2006 | Slide 4 of 20 STOP

Documentation What is being made? Most of us when attempting a task need some sort of documentation Module 12 – part 1 January 2006 | Slide 5 of 20 STOP

Documentation And if the drawing is wrong! Module 12 – part 1 January 2006 | Slide 6 of 20 STOP

Documentation Module 12 – part 1 January 2006 | Slide 7 of 20 STOP

Documentation Why are documents so important? l Communication l Cost l Audit trail Module 12 – part 1 January 2006 | Slide 8 of 20 STOP

Documentation General Principles – I l Documents should be ä designed ä prepared ä reviewed ä distributed with care l Comply with marketing authorization l Design of documentation important 15. 2 Module 12 – part 1 January 2006 | Slide 9 of 20 STOP

Documentation General Principles – II l Look at the “Style” of the document ä Instructions in the imperative ä Short sentences preferred to long sentences l Approval of documentation ä Approved, signed and dated by appropriate responsible persons ä No document should be changed without authorization and approval 15. 3 Module 12 – part 1 January 2006 | Slide 10 of 20 STOP

Documentation General Principles – III l Contents of documents should be clear (easy to understand) and include, e. g. ä Title, nature, objective or purpose l Layout in orderly fashion l Easy to be filled in and checked l Clear and readable – including copies made l No errors if master documents are copied for working documents 15. 4 Module 12 – part 1 January 2006 | Slide 11 of 20 STOP
Documentation General Principles – IV Documentation control l Regular review of documents l Kept up to date (current) - amended l Superseded documents removed and not used ä Distribution and retrieval of documentation l Retention time for superseded documents 15. 5 Module 12 – part 1 January 2006 | Slide 12 of 20 STOP

Documentation General Principles – V Data entry l Clear, readable and indelible l Design to allow for sufficient space for entries l Changes to entries: ä signed, dated and reason given ä original entry still readable l Entries at the time of action l All significant actions recorded – traceable Module 12 – part 1 January 2006 | Slide 13 of 20 STOP 15. 6 – 15. 8
Documentation General Principles – VI Data entry (cont. ) l Electronic data processing systems, photographic systems or other reliable means l Systems require SOPs and records l Accuracy of records checked l Authorized persons - access and changes l Password controlled l Entries checked Module 12 – part 1 January 2006 | Slide 14 of 20 15. 9 STOP

Documentation General Principles – VII Data entry (cont. ) l Batch records stored electronically: ä Protected ä Back-up transfer, e. g. magnetic tape, microfilm, paper print-outs l Records kept 1 year after expiry date of product l Data readily available during retention period 15. 9 Module 12 – part 1 January 2006 | Slide 15 of 20 STOP
Documentation Types of Documentation l Labels l Specifications and testing procedures l Master formulae and instructions l Batch processing and batch packaging records l Standard Operating Procedures (SOPs) l Records ä Stock control and distribution records l Other documents … Module 12 – part 1 January 2006 | Slide 16 of 20 STOP
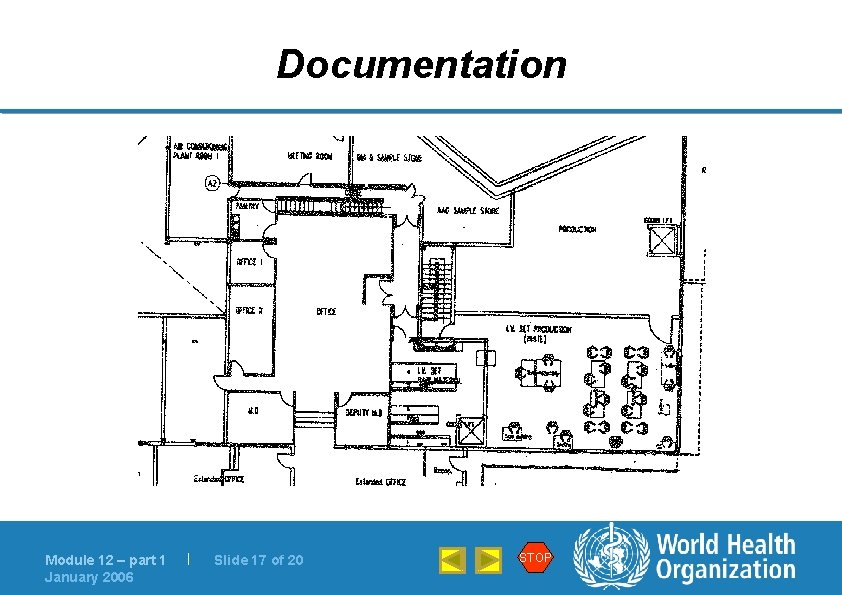
Documentation Module 12 – part 1 January 2006 | Slide 17 of 20 STOP

Documentation l Photographs can be documents and part of a herbal identification, provided they are properly authorized and controlled Module 12 – part 1 January 2006 | Slide 18 of 20 STOP
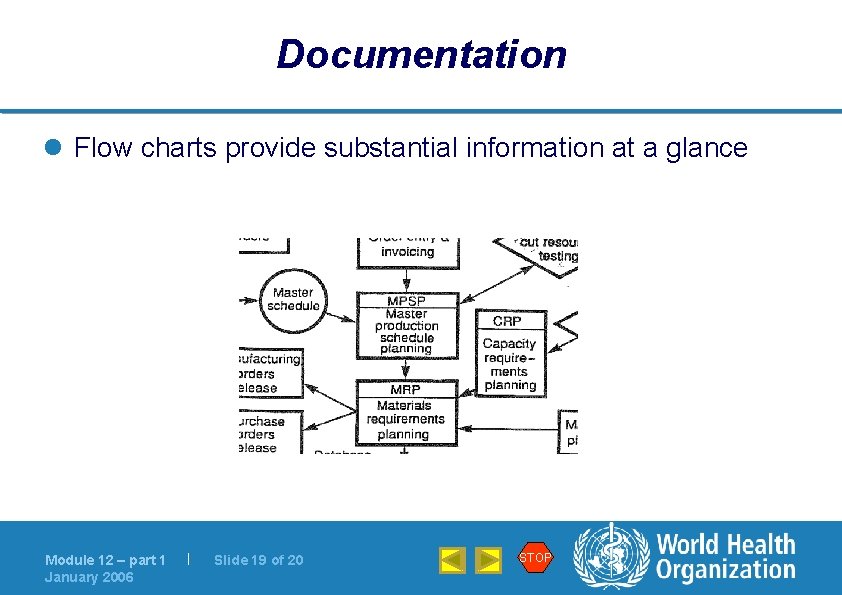
Documentation l Flow charts provide substantial information at a glance Module 12 – part 1 January 2006 | Slide 19 of 20 STOP

Documentation Types of Documentation l The different types of documents will be discussed in detail in Documentation: Part 2 Module 12 – part 1 January 2006 | Slide 20 of 20 STOP
- Slides: 20