BASIC PRINCIPLES OF ELECTRODE PROCESSES Heterogeneous kinetics I

BASIC PRINCIPLES OF ELECTRODE PROCESSES Heterogeneous kinetics I
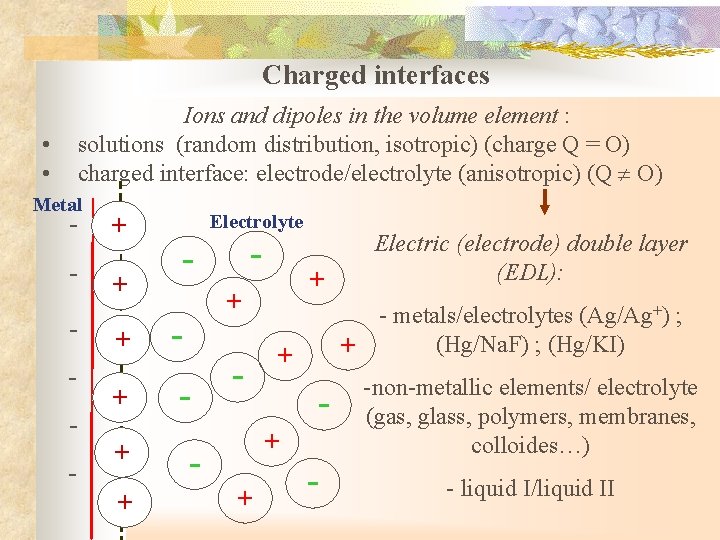
Charged interfaces Ions and dipoles in the volume element : solutions (random distribution, isotropic) (charge Q = O) charged interface: electrode/electrolyte (anisotropic) (Q O) • • Metal - + - + + Electrolyte - - + + - - - metals/electrolytes (Ag/Ag+) ; (Hg/Na. F) ; (Hg/KI) + + Electric (electrode) double layer (EDL): - -non-metallic elements/ electrolyte (gas, glass, polymers, membranes, colloides…) - liquid I/liquid II
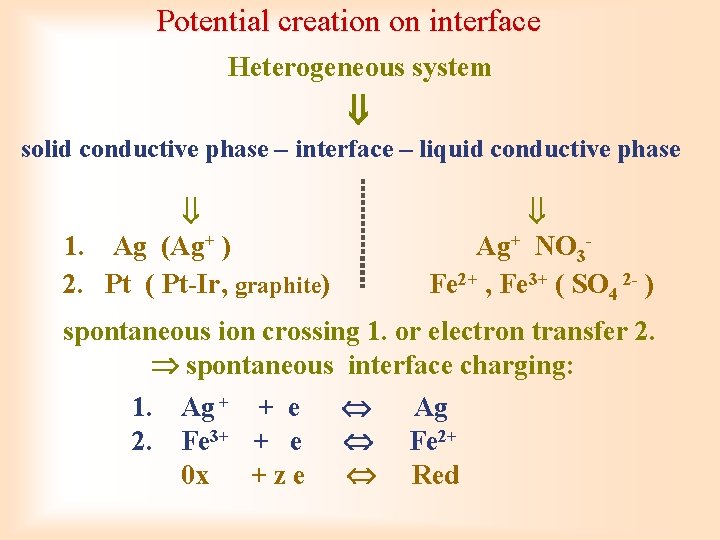
Potential creation on interface Heterogeneous system solid conductive phase – interface – liquid conductive phase 1. Ag (Ag+ ) Ag+ NO 3 - 2. Pt ( Pt-Ir, graphite) Fe 2+ , Fe 3+ ( SO 4 2 - ) spontaneous ion crossing 1. or electron transfer 2. spontaneous interface charging: 1. Ag + + e Ag 2. Fe 3+ + e Fe 2+ 0 x + z e Red
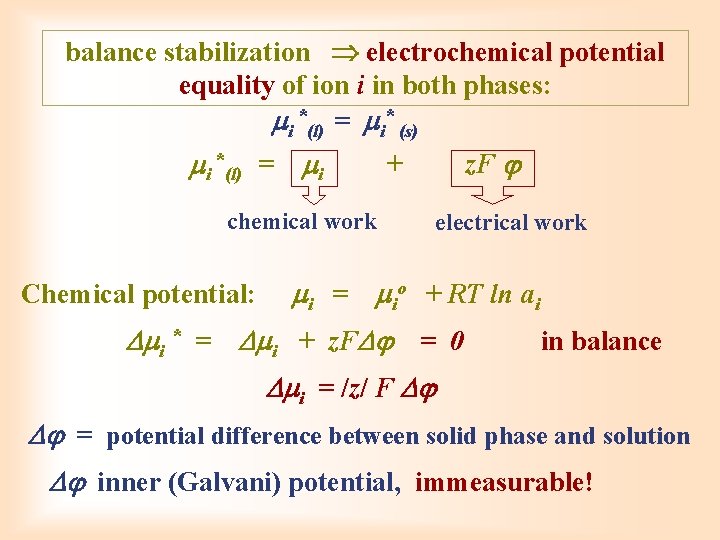
balance stabilization electrochemical potential equality of ion i in both phases: i *(l) = i* (s) i *(l) = i + chemical work Chemical potential: i = z. F electrical work io + RT ln ai i * = i + z. F = 0 in balance i = /z/ F = potential difference between solid phase and solution inner (Galvani) potential, immeasurable!

Measurable cell potential EMN , made from 2 hemi-cells: standard hydrogen electrode SHE Eo = 0 V measured electrode E = b + EMN = E - 0 = E Nernst : 1. 2. That was a balance b = constant
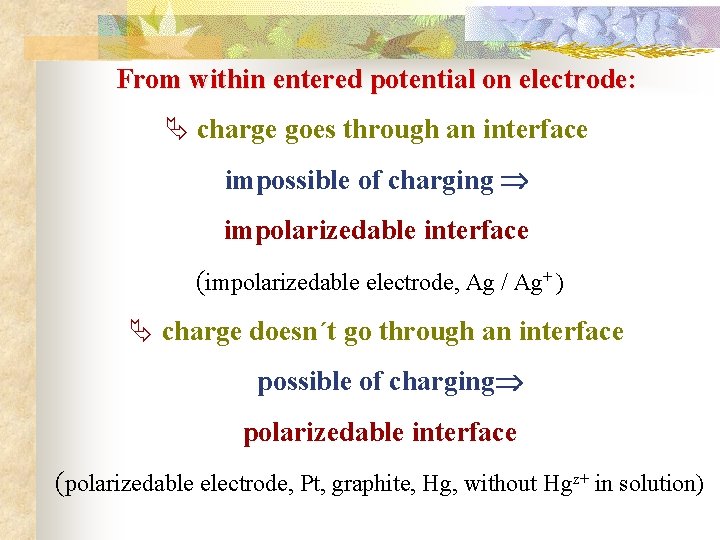
From within entered potential on electrode: Ä charge goes through an interface impossible of charging impolarizedable interface (impolarizedable electrode, Ag / Ag+ ) Ä charge doesn´t go through an interface possible of charging polarizedable interface (polarizedable electrode, Pt, graphite, Hg, without Hgz+ in solution)

ELECTRODE DOUBLE LAYER (EDL)

ELECTRODE DOUBLE LAYER (EDL) electrostatic potential charge density σ surface tension g capacity C adsorption G Electrode +σS - + φ Solution -σM +Solution - + + - Electrode - G + + + - Solution + + + -
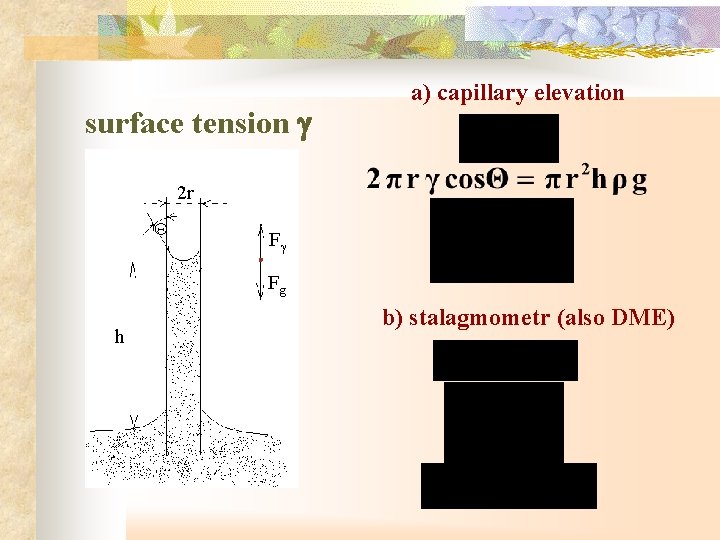
surface tension g a) capillary elevation 2 r F Fg h b) stalagmometr (also DME)
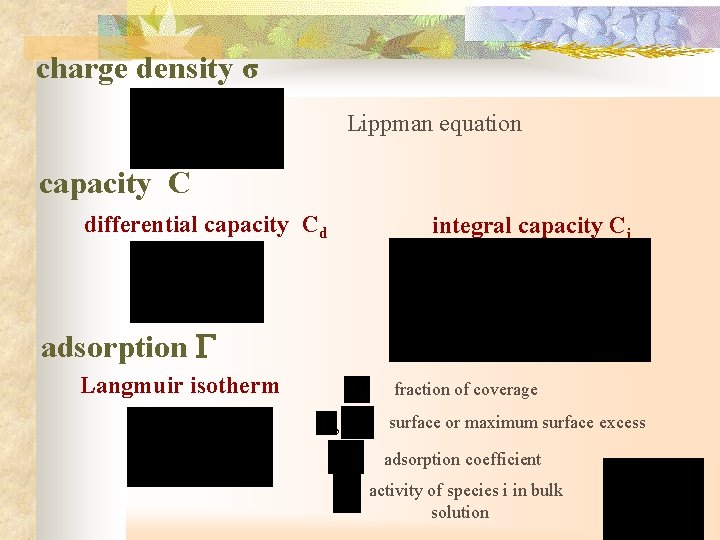
charge density σ Lippman equation capacity C differential capacity Cd integral capacity Ci adsorption G Langmuir isotherm fraction of coverage , surface or maximum surface excess adsorption coefficient activity of species i in bulk solution
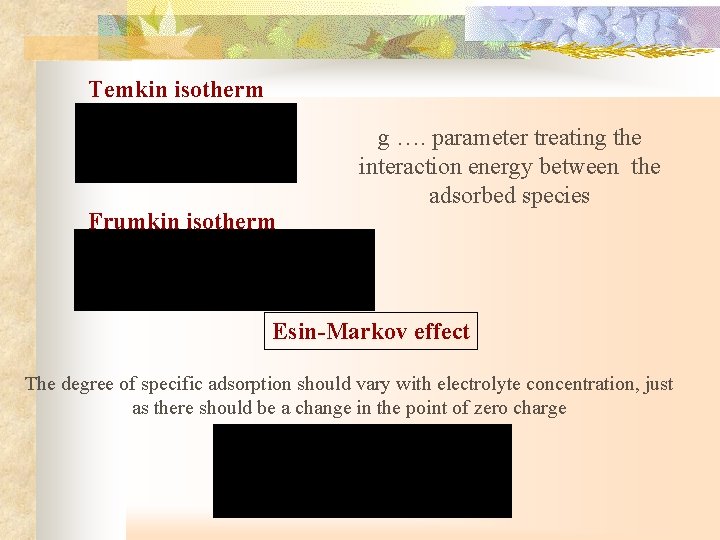
Temkin isotherm Frumkin isotherm g …. parameter treating the interaction energy between the adsorbed species Esin-Markov effect The degree of specific adsorption should vary with electrolyte concentration, just as there should be a change in the point of zero charge
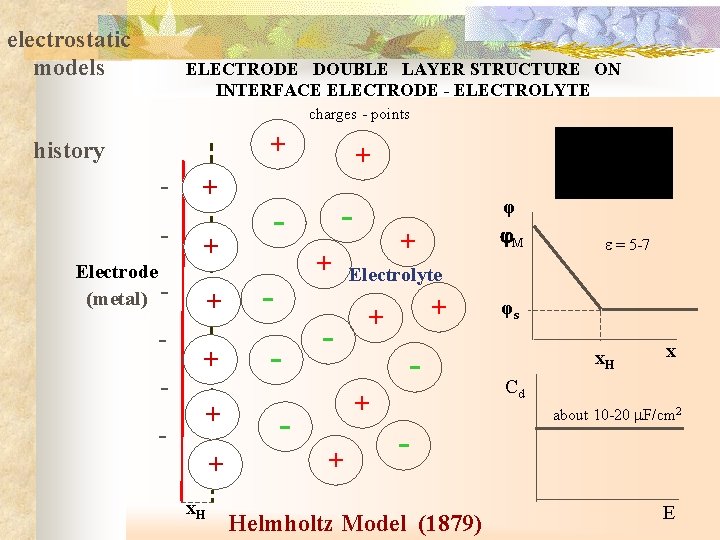
electrostatic models ELECTRODE DOUBLE LAYER STRUCTURE ON INTERFACE ELECTRODE - ELECTROLYTE charges - points + history - + Electrode (metal) - - + + x. H + - - + + Electrolyte + + - - + + - Helmholtz Model (1879) φ M = 5 -7 φs x. H x Cd about 10 -20 F/cm 2 E
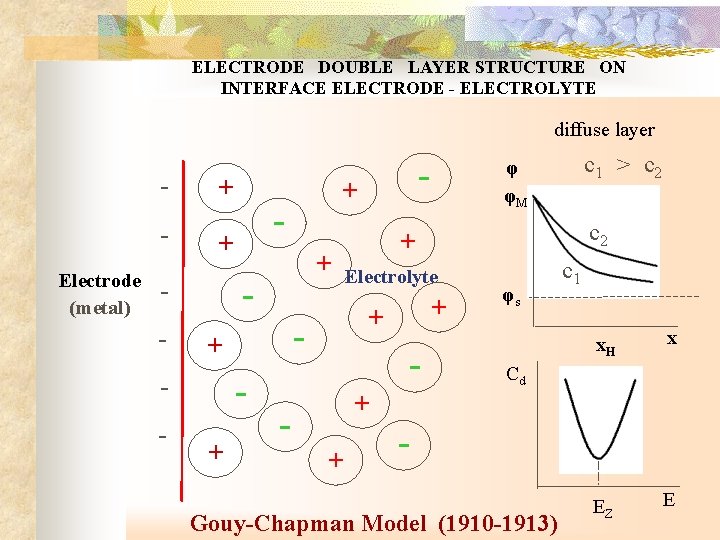
ELECTRODE DOUBLE LAYER STRUCTURE ON INTERFACE ELECTRODE - ELECTROLYTE diffuse layer Electrode (metal) - + - - + c 1 > c 2 φ φM c 2 + - + - - - + Electrolyte + + - φs c 1 x. H x EZ E Cd + + - Gouy-Chapman Model (1910 -1913)
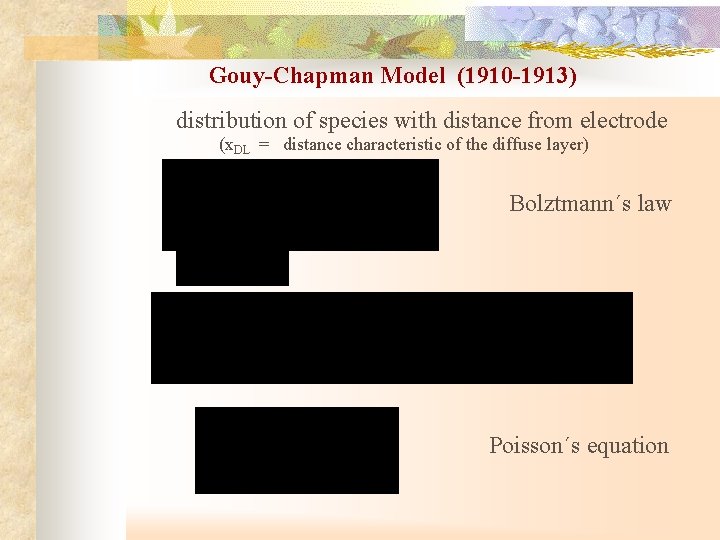
Gouy-Chapman Model (1910 -1913) distribution of species with distance from electrode (x. DL = distance characteristic of the diffuse layer) Bolztmann´s law Poisson´s equation
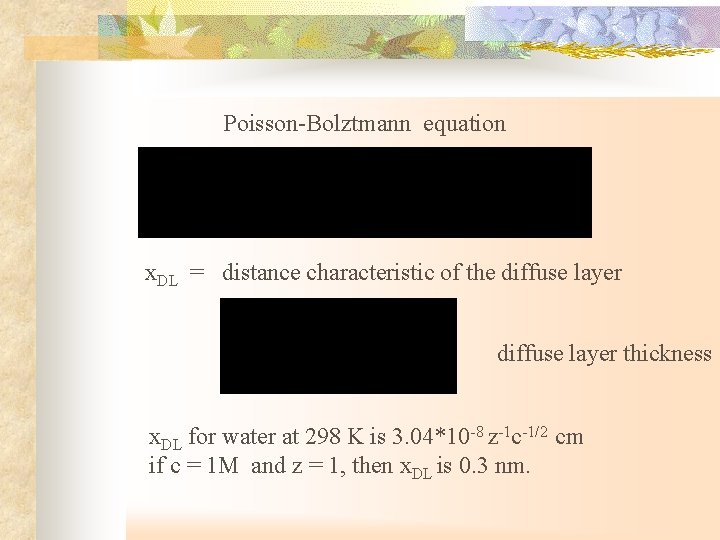
Poisson-Bolztmann equation x. DL = distance characteristic of the diffuse layer thickness x. DL for water at 298 K is 3. 04*10 -8 z-1 c-1/2 cm if c = 1 M and z = 1, then x. DL is 0. 3 nm.

ELECTRODE DOUBLE LAYER STRUCTURE ON INTERFACE ELECTRODE - ELECTROLYTE Compact Layer + Electrode (metal) + + - + + Electrolyte + + - - + + φ φM Diffuse Layer φs x. H Cd x OHP - Stern Model (1924) EZ E

Stern Model (1924) close to EZ, CH >> CGC and so Cd ~ CGC far from EZ, CH CGC and so Cd ~ CH separation plane between the two zones is called the outer Helmholtz plane (OHP)
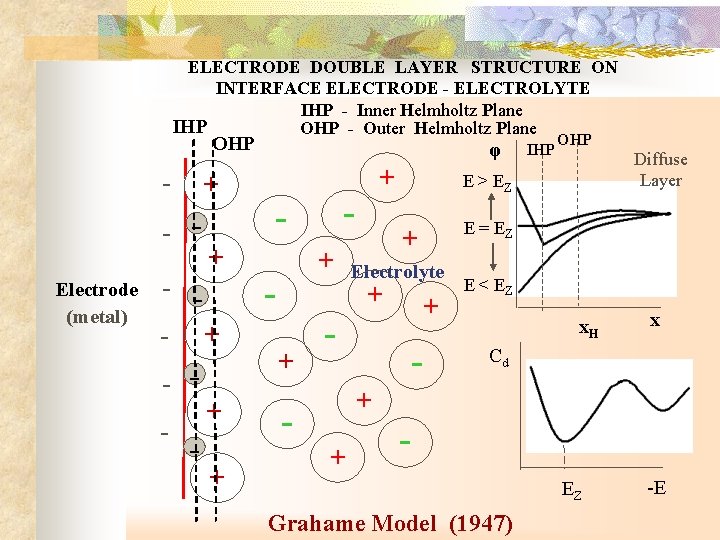
ELECTRODE DOUBLE LAYER STRUCTURE ON INTERFACE ELECTRODE - ELECTROLYTE IHP - Inner Helmholtz Plane IHP OHP - Outer Helmholtz Plane OHP - + - Electrode (metal) - + + - - + - φ + + - OHP E > EZ + Electrolyte + + - IHP - Diffuse Layer E = EZ E < EZ x. H x Cd + + EZ Grahame Model (1947) -E
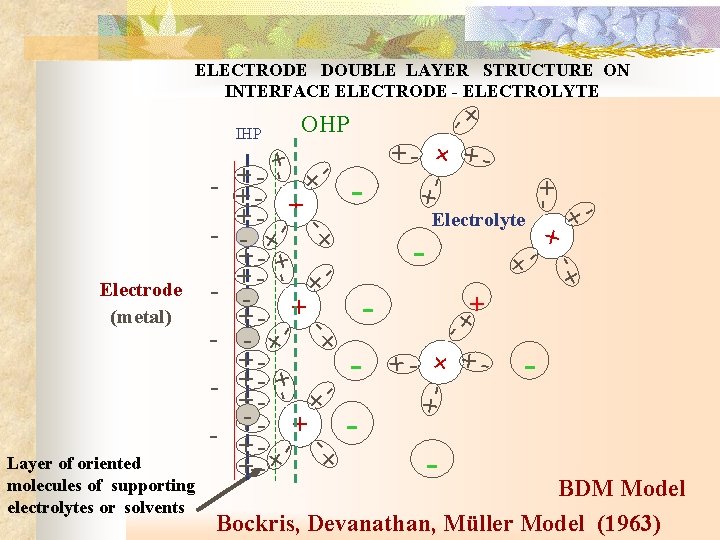
ELECTRODE DOUBLE LAYER STRUCTURE ON INTERFACE ELECTRODE - ELECTROLYTE - + - + - + + + - + - - + + + + - Layer of oriented molecules of supporting electrolytes or solvents - + + - - - Electrolyte + - - - + - Electrode (metal) + - + + + + + - + - - + - OHP IHP - BDM Model Bockris, Devanathan, Müller Model (1963)
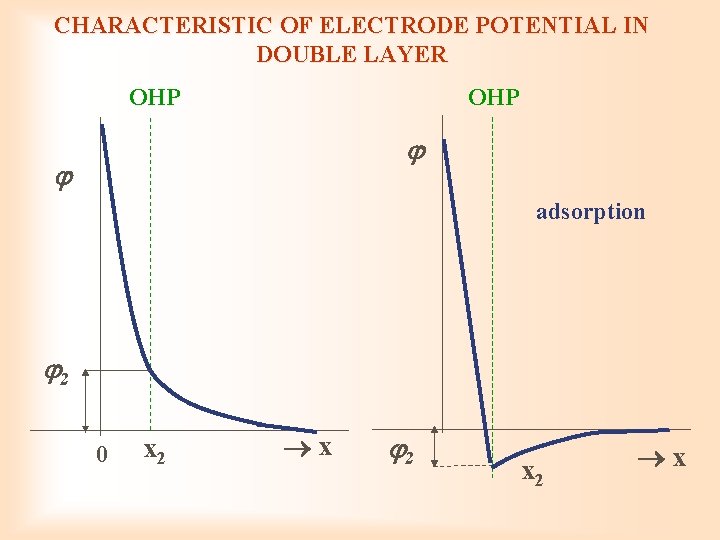
CHARACTERISTIC OF ELECTRODE POTENTIAL IN DOUBLE LAYER OHP adsorption 2 0 x 2 x 2 x 2 x
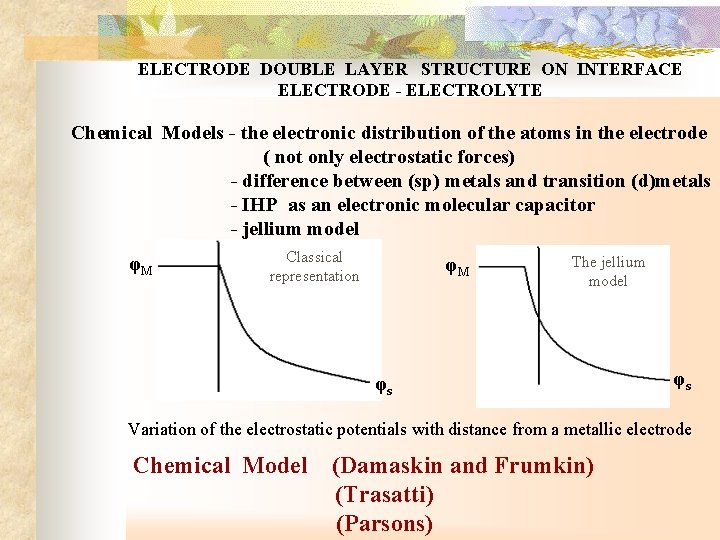
ELECTRODE DOUBLE LAYER STRUCTURE ON INTERFACE ELECTRODE - ELECTROLYTE Chemical Models - the electronic distribution of the atoms in the electrode ( not only electrostatic forces) - difference between (sp) metals and transition (d)metals - IHP as an electronic molecular capacitor - jellium model φM Classical representation φM The jellium model φs φs Variation of the electrostatic potentials with distance from a metallic electrode Chemical Model (Damaskin and Frumkin) (Trasatti) (Parsons)
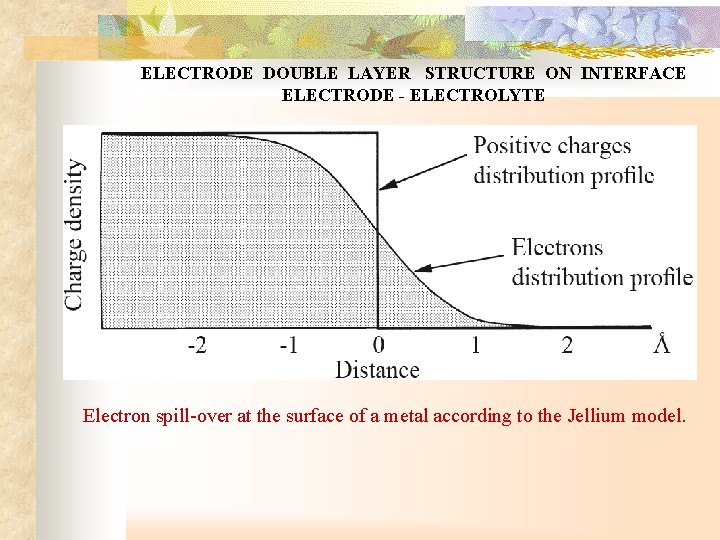
ELECTRODE DOUBLE LAYER STRUCTURE ON INTERFACE ELECTRODE - ELECTROLYTE Electron spill-over at the surface of a metal according to the Jellium model.
- Slides: 22