Basic Principles of Chemistry Unit 2 Chemical Reactions
Basic Principles of Chemistry Unit 2: Chemical Reactions and Their Significance Lecture #11

Precipitation Reactions • A solid substance is formed in a solution where there was not solid previously

Solutions • SOLUTE dissolves in SOLVENT to form SOLUTION • Solute is SOLUBLE in solvent
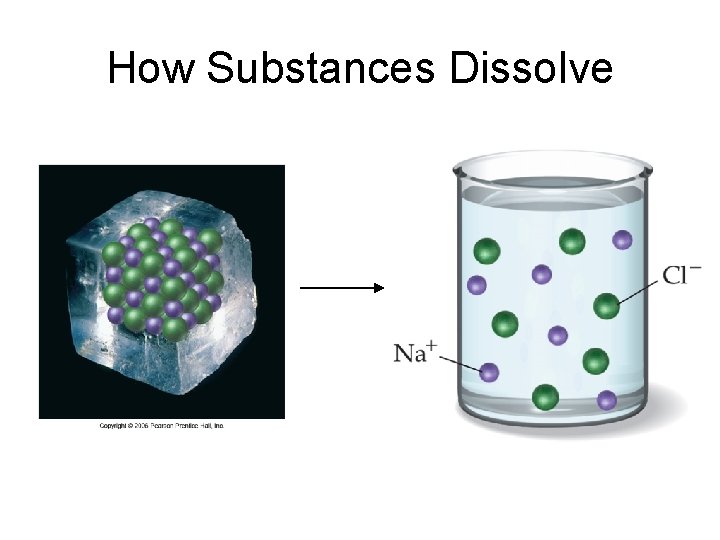
How Substances Dissolve
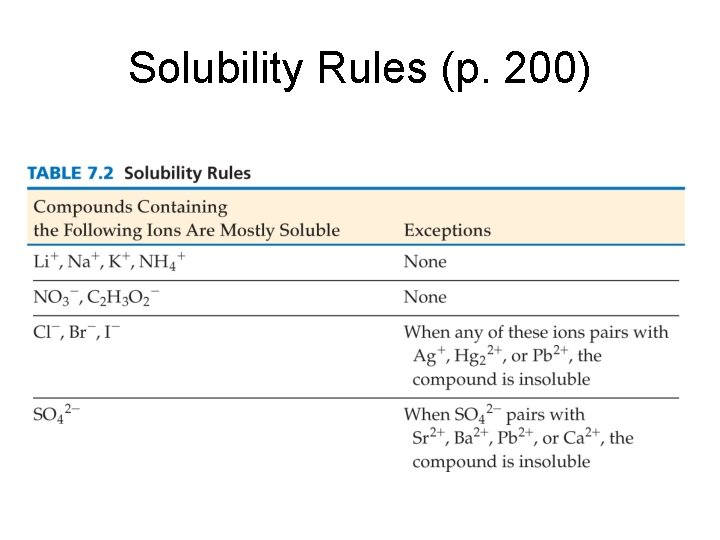
Solubility Rules (p. 200)
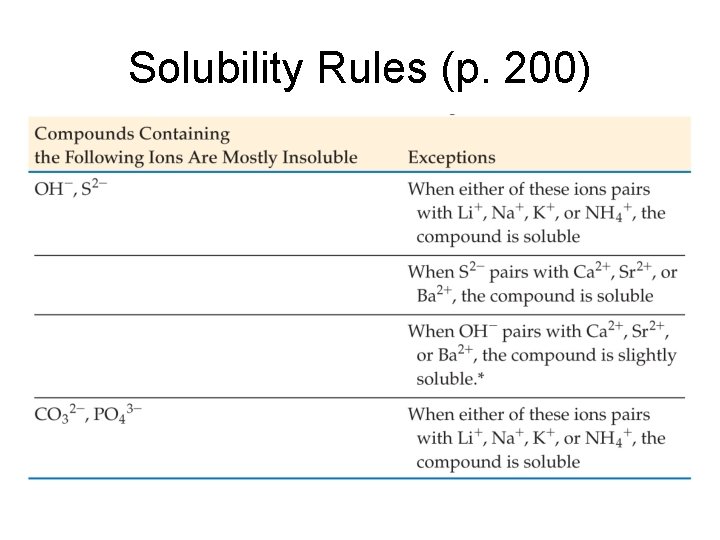
Solubility Rules (p. 200)
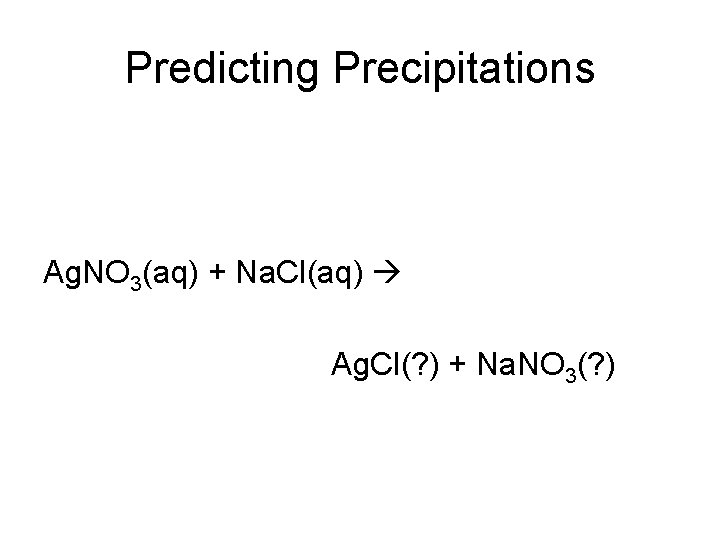
Predicting Precipitations Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(? ) + Na. NO 3(? )
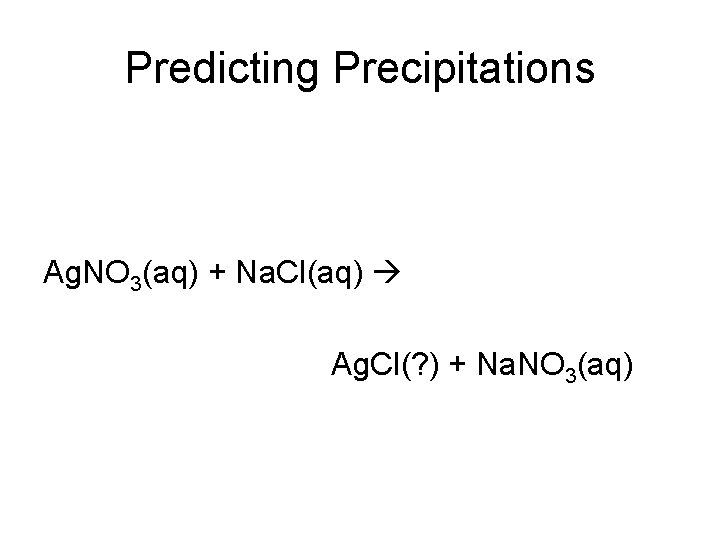
Predicting Precipitations Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(? ) + Na. NO 3(aq)
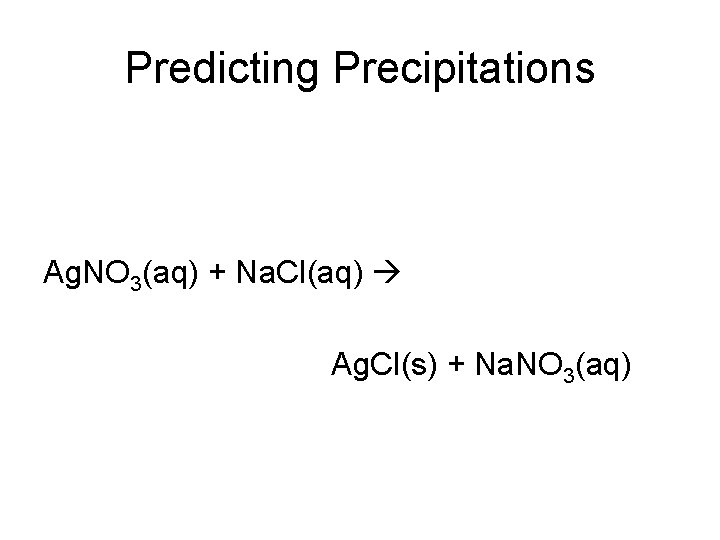
Predicting Precipitations Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(s) + Na. NO 3(aq)
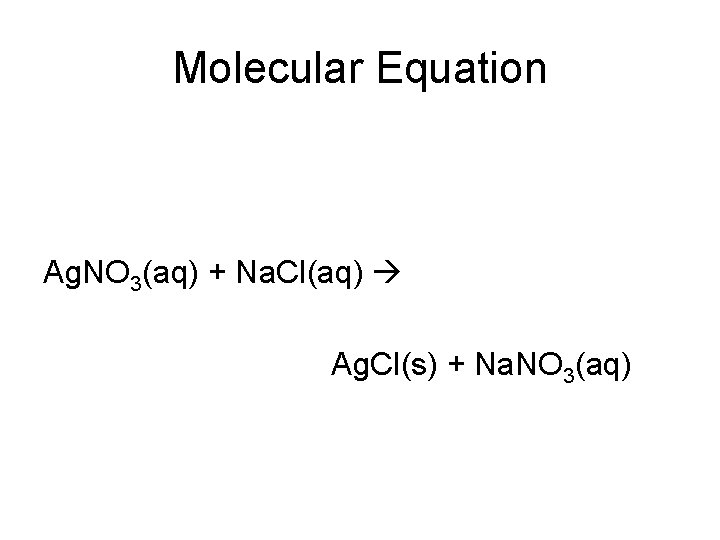
Molecular Equation Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(s) + Na. NO 3(aq)
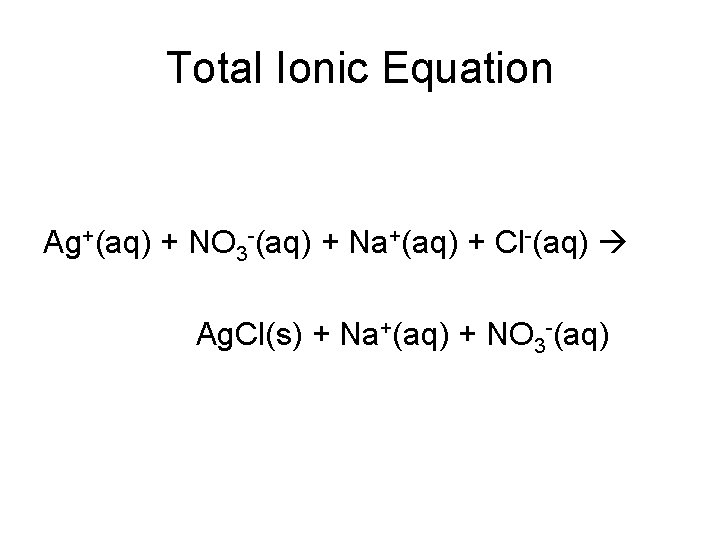
Total Ionic Equation Ag+(aq) + NO 3 -(aq) + Na+(aq) + Cl-(aq) Ag. Cl(s) + Na+(aq) + NO 3 -(aq)
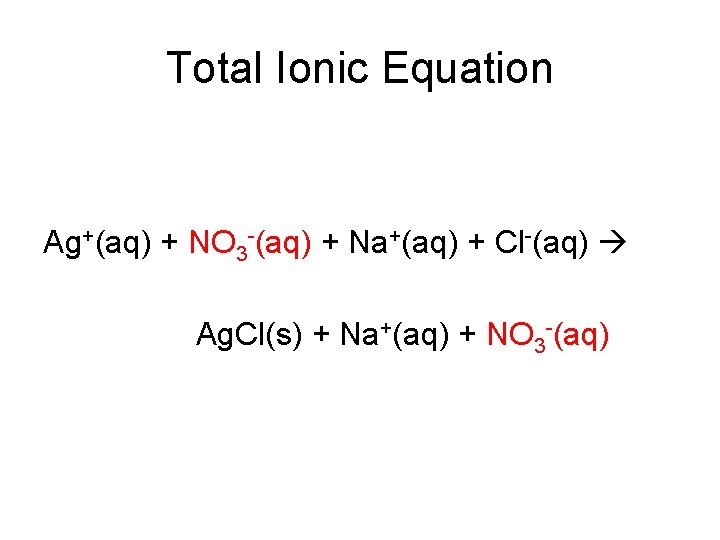
Total Ionic Equation Ag+(aq) + NO 3 -(aq) + Na+(aq) + Cl-(aq) Ag. Cl(s) + Na+(aq) + NO 3 -(aq)
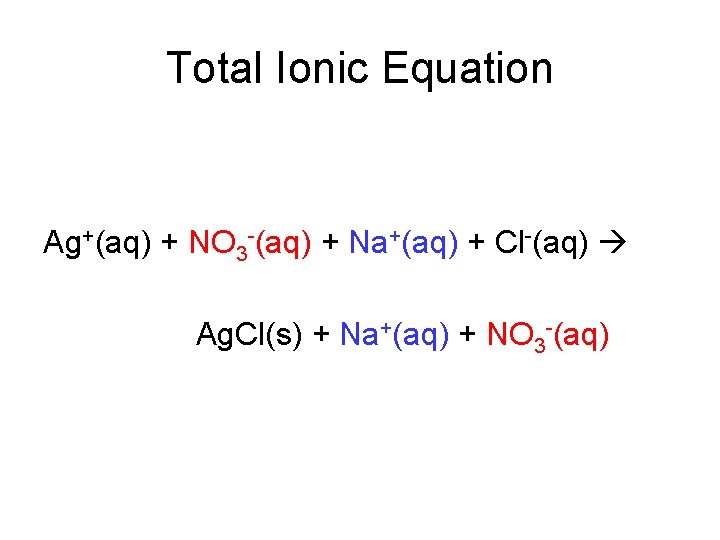
Total Ionic Equation Ag+(aq) + NO 3 -(aq) + Na+(aq) + Cl-(aq) Ag. Cl(s) + Na+(aq) + NO 3 -(aq)
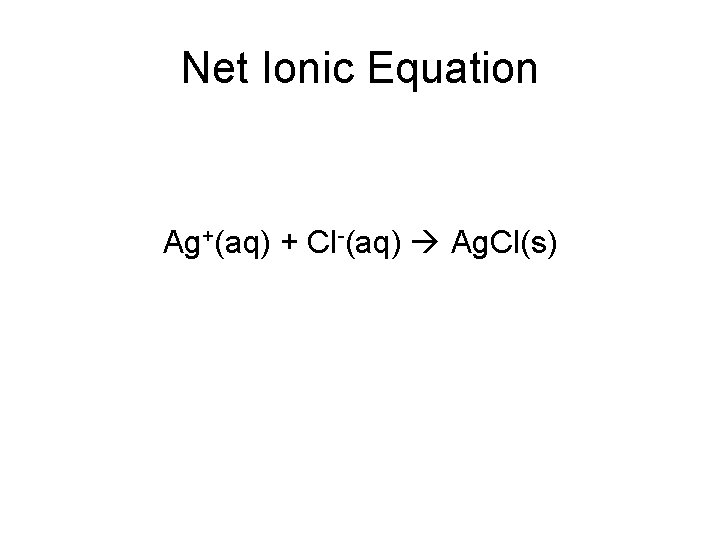
Net Ionic Equation Ag+(aq) + Cl-(aq) Ag. Cl(s)
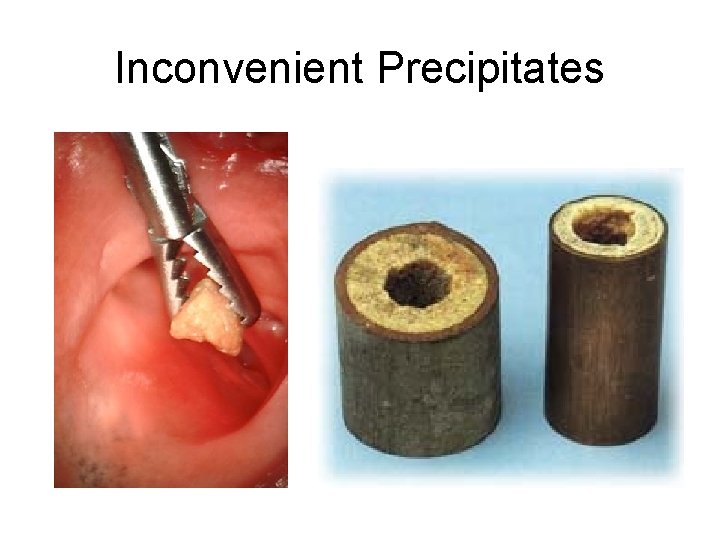
Inconvenient Precipitates
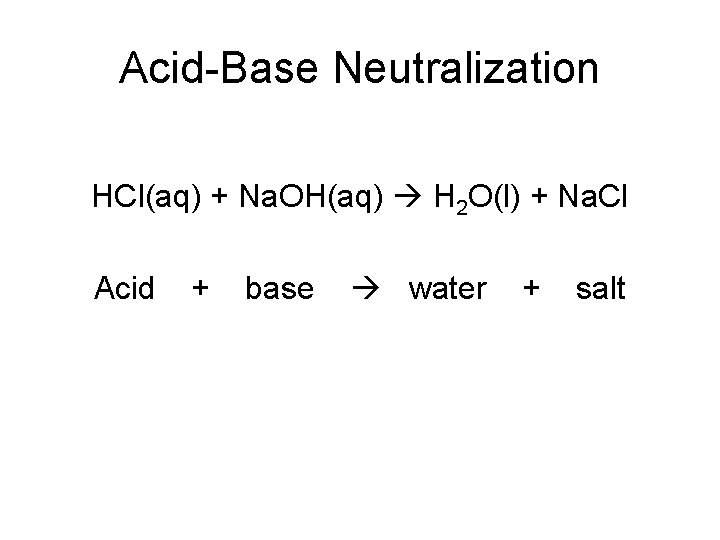
Acid-Base Neutralization HCl(aq) + Na. OH(aq) H 2 O(l) + Na. Cl Acid + base water + salt

Acid-Base Neutralization HC 2 H 3 O 2(aq) + Na. HCO 3(aq) H 2 O(l) + Na. C 2 H 3 O 2(aq) + CO 2(g)
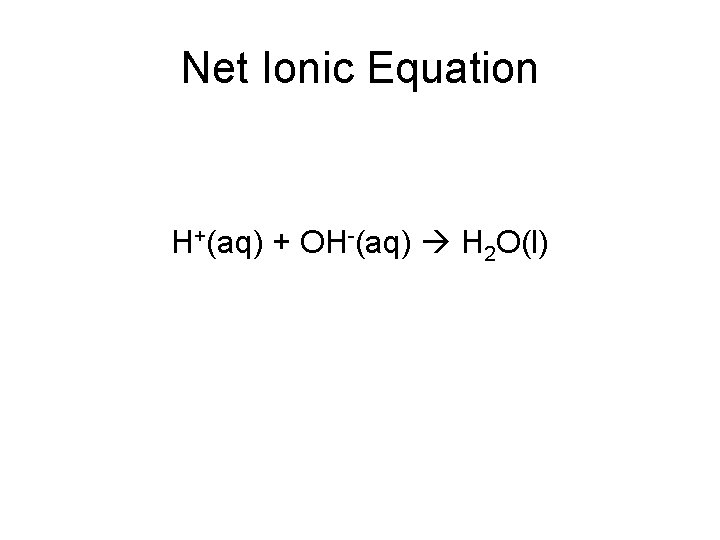
Net Ionic Equation H+(aq) + OH-(aq) H 2 O(l)

TUMS HCl(aq) + Ca. CO 3(aq) H 2 O(l) + Ca. Cl 2(aq) + CO 2(g)
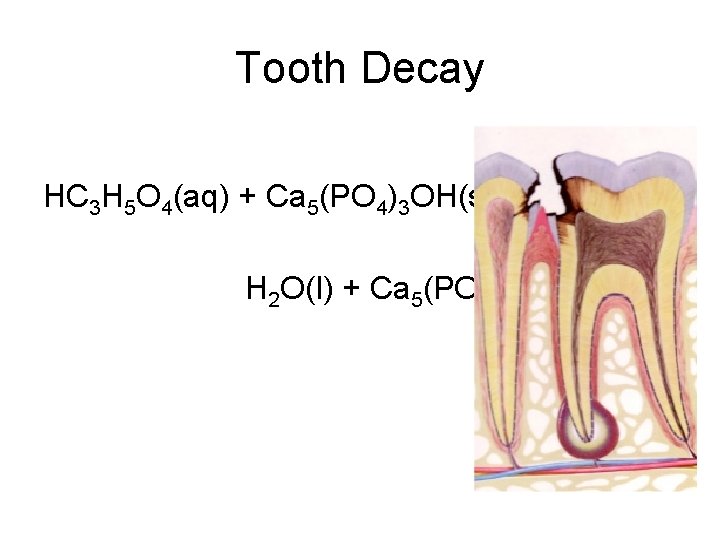
Tooth Decay HC 3 H 5 O 4(aq) + Ca 5(PO 4)3 OH(s) H 2 O(l) + Ca 5(PO 4)3 C 3 H 5 O 4(aq)

Dissolving Scale Ca. CO 3(s) + 2 HCl(aq) Ca. Cl 2 + H 2 O(l) + CO 2(g)

Oxidation-Reduction Al(s) + 6 HCl(aq) 2 Al. Cl 3(aq) + 3 H 2(g)
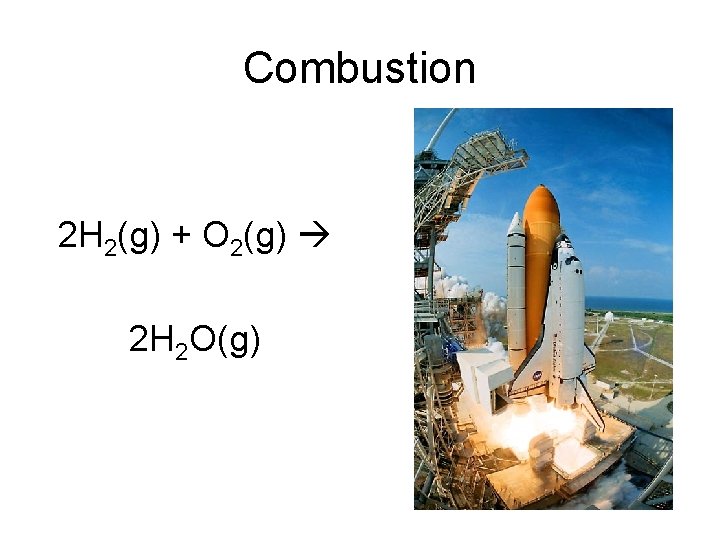
Combustion 2 H 2(g) + O 2(g) 2 H 2 O(g)
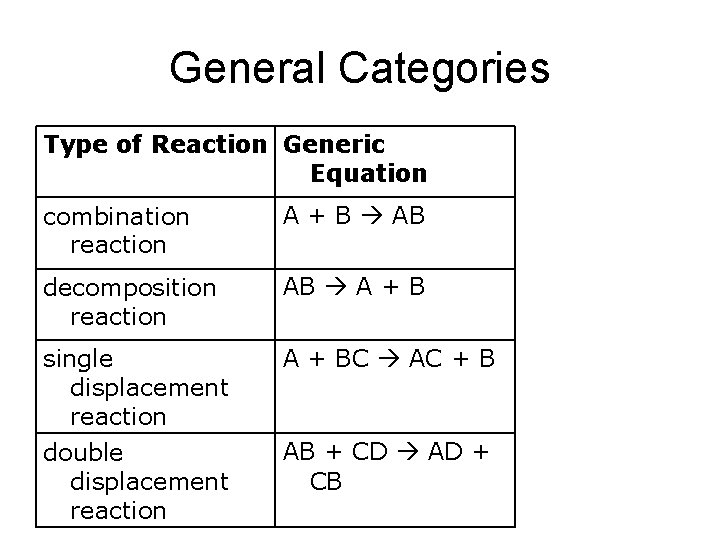
General Categories Type of Reaction Generic Equation combination reaction A + B AB decomposition reaction AB A + B single displacement reaction A + BC AC + B double displacement reaction AB + CD AD + CB
- Slides: 24