Basic Palliative Care for District Hospital Team Subcutaneous

Basic Palliative Care for District Hospital Team Subcutaneous Infusion and Administration of Drug via Syringe Driver ������������������������
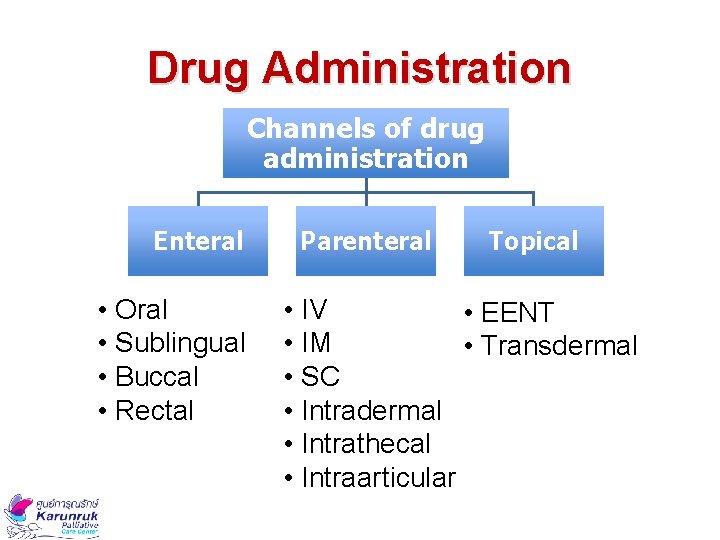
Drug Administration Channels of drug administration Enteral • Oral • Sublingual • Buccal • Rectal Parenteral Topical • IV • EENT • IM • Transdermal • SC • Intradermal • Intrathecal • Intraarticular
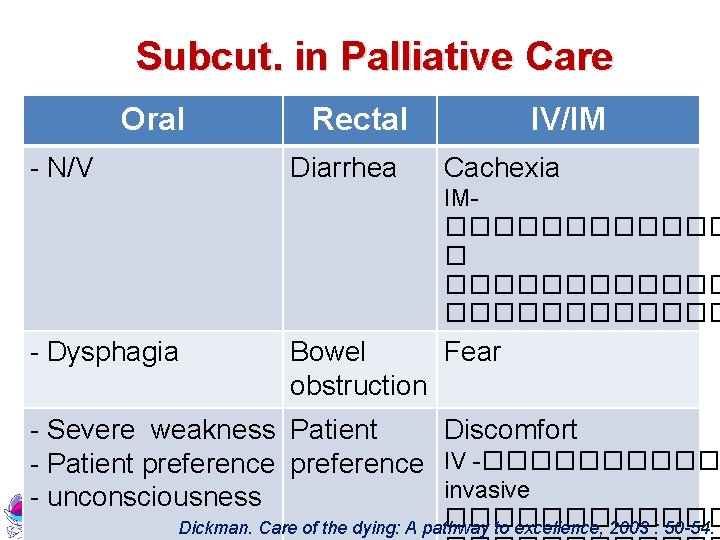
Subcut. in Palliative Care Oral - N/V Rectal Diarrhea IV/IM Cachexia IM������ � ������������ - Dysphagia Bowel Fear obstruction - Severe weakness Patient Discomfort - Patient preference IV -����� invasive - unconsciousness ������ Dickman. Care of the dying: A pathway to excellence, 2003 : 50 -54.
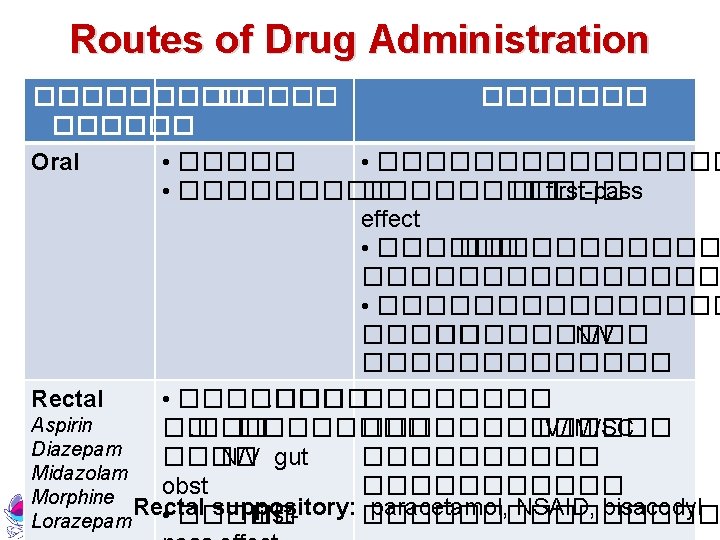
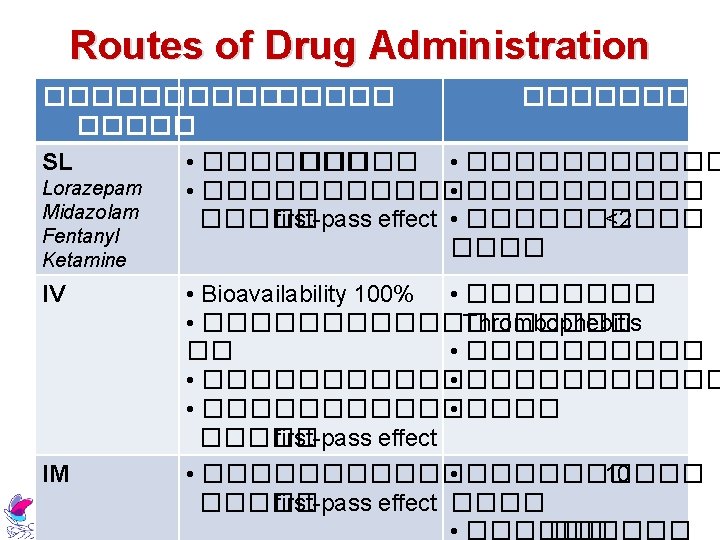
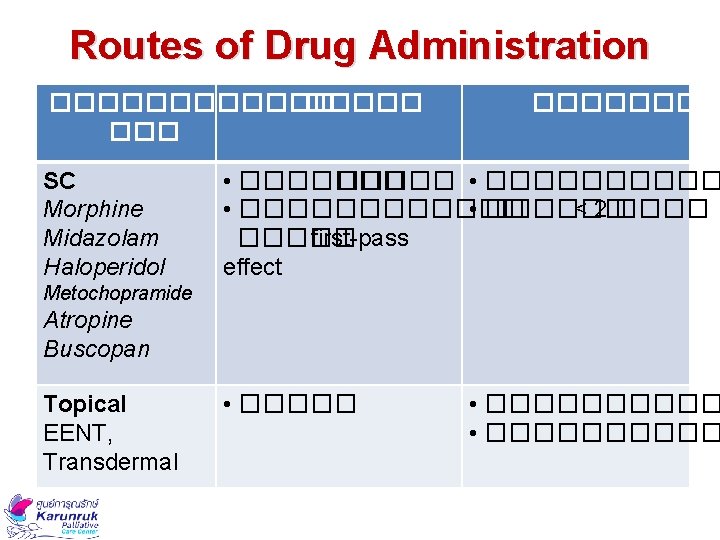
Routes of Drug Administration ������ SC Morphine Midazolam Haloperidol ������� • ���������� • ������ < 2 ����� first-pass effect Metochopramide Atropine Buscopan Topical EENT, Transdermal • ���������� • �����
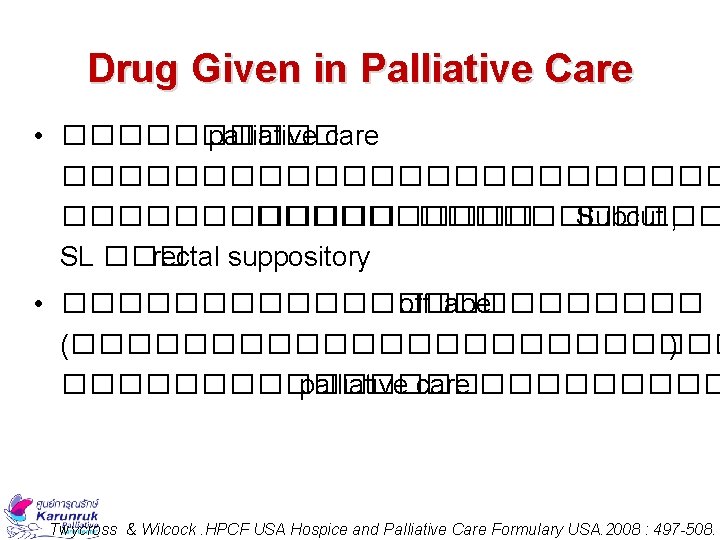
Drug Given in Palliative Care • ����� palliative care ������������ ����������� Subcut. , SL ��� rectal suppository • ������������ off label (������������ ) �������� palliative care ����� Twycross & Wilcock. HPCF USA Hospice and Palliative Care Formulary USA. 2008 : 497 -508.
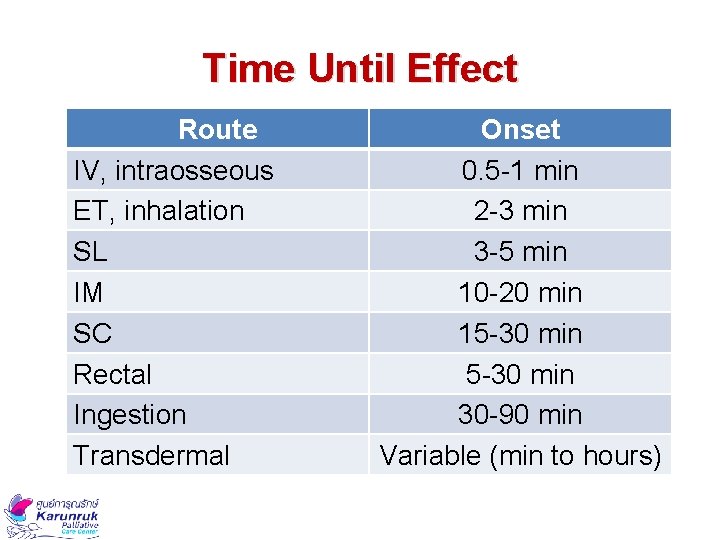
Time Until Effect Route IV, intraosseous ET, inhalation SL IM SC Rectal Ingestion Transdermal Onset 0. 5 -1 min 2 -3 min 3 -5 min 10 -20 min 15 -30 min 30 -90 min Variable (min to hours)
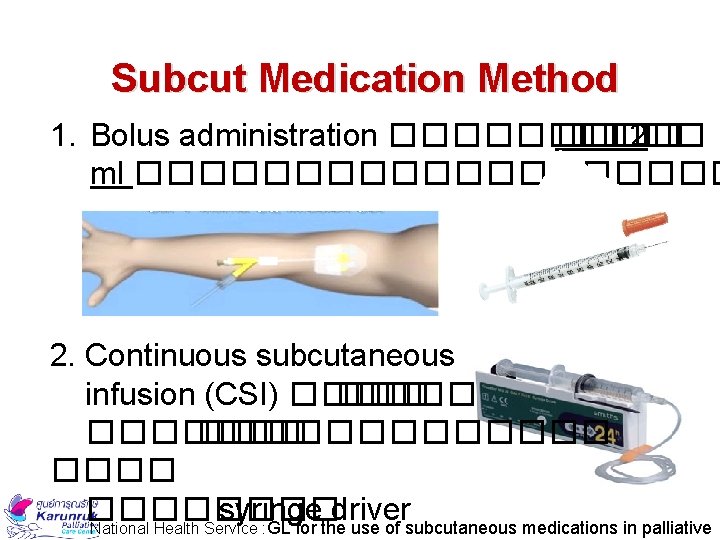
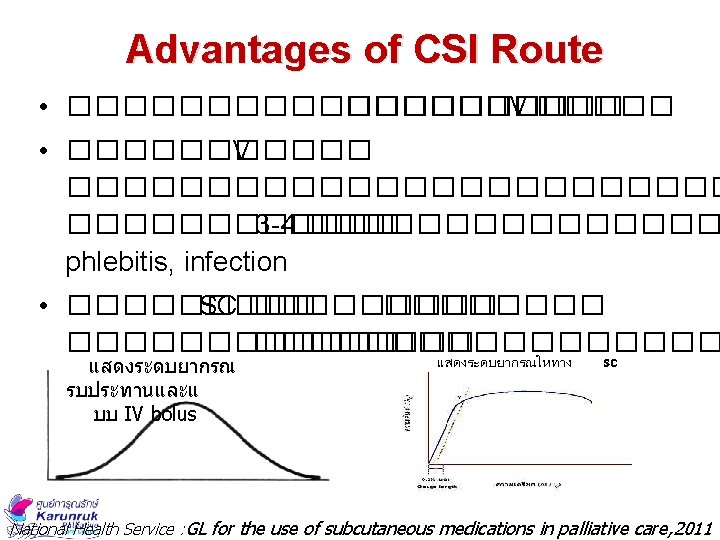
Subcut Medication Method 1. Bolus administration ����� 2 ml ���������� 2. Continuous subcutaneous infusion (CSI) ��� �������� �������� syringe driver National Health Service : GL for the use of subcutaneous medications in palliative

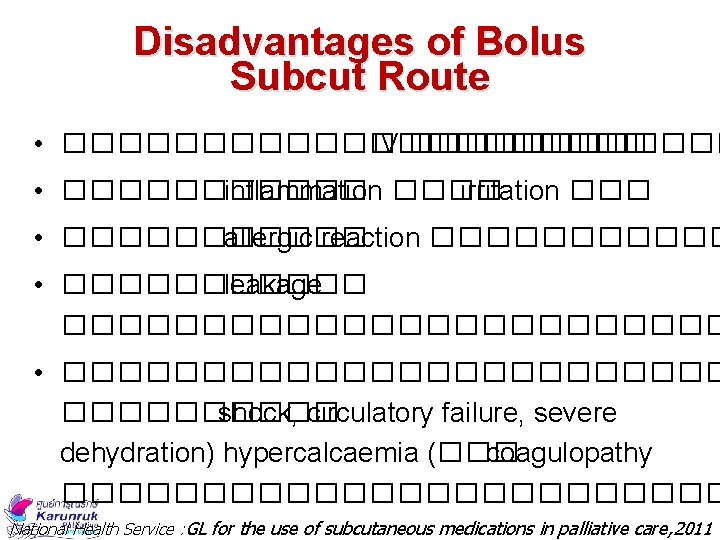
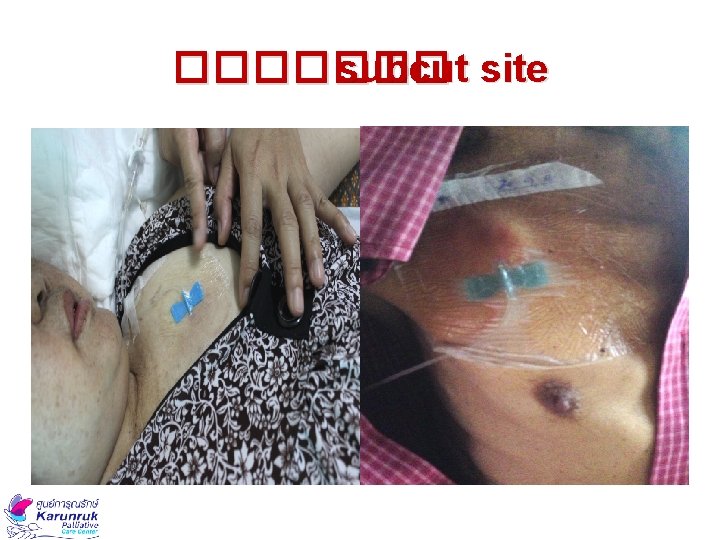
Disadvantages of Bolus Subcut Route • ����������� IV ������ • ������ inflammation ���� irritation ��� • ������ allergic reaction ������ • ������ leakage ������������ • ������������ shock, circulatory failure, severe dehydration) hypercalcaemia (��� coagulopathy ������������ National Health Service : GL for the use of subcutaneous medications in palliative care, 2011
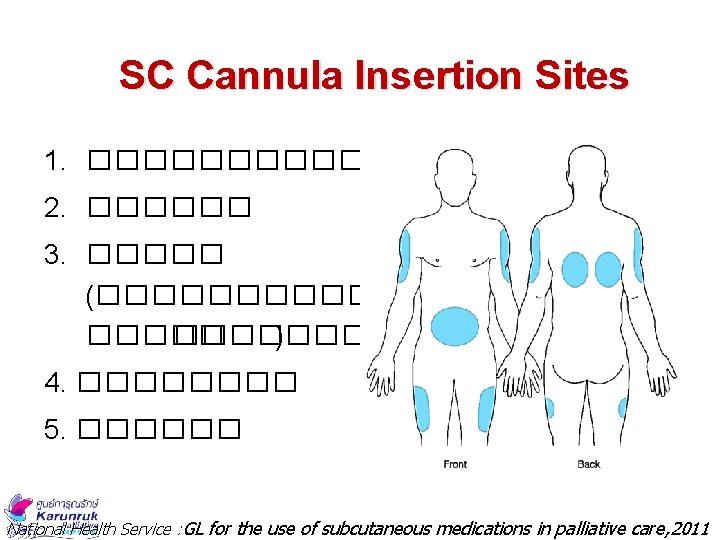
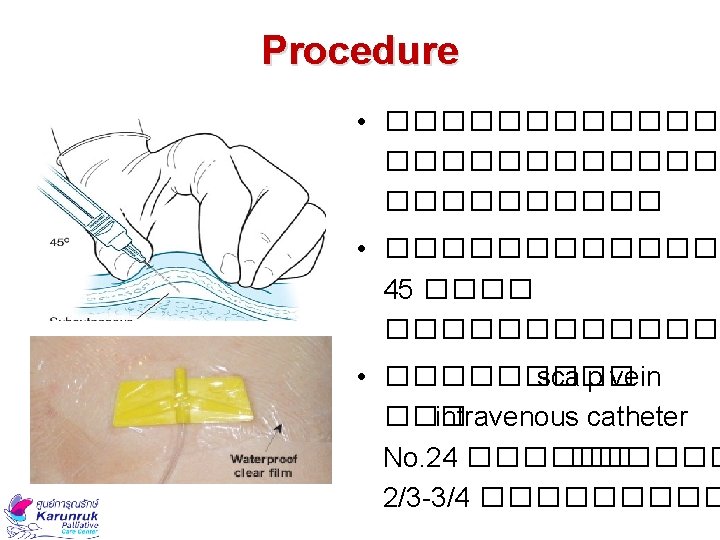
SC Cannula Insertion Sites 1. ������� 2. ������ 3. ����� (�������� ) 4. ���� 5. ������ National Health Service : GL for the use of subcutaneous medications in palliative care, 2011

Sites Not Suitable for Injection • �������� lymphedema • ���������� ascites • ����������� • ������������ • ������������ • ���������������� National Health Service : GL for the use of subcutaneous medications in palliative care, 2011
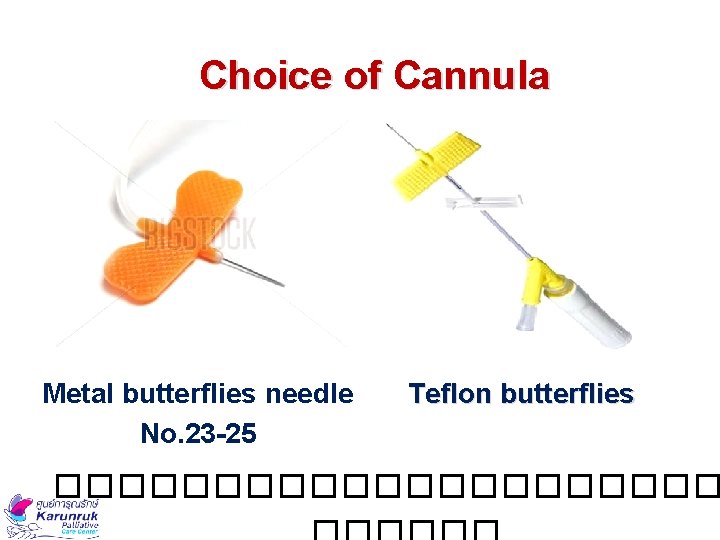
Choice of Cannula Metal butterflies needle No. 23 -25 Teflon butterflies �����������

Subcut Drug Administration in PC • • • Atropine Dexamethazone Fentanyl Haloperidol Hyoscine butylbromide (Busopan) • Ketamine • Ketololac • • Morphine Midazolam Metoclopramide Octreotide Ondansetron Phenobarbitone Ranitidine Omeprazole Twycross & Wilcock. HPCF USA Hospice & Palliative Care Formulary USA. , 2008: 497 -508.
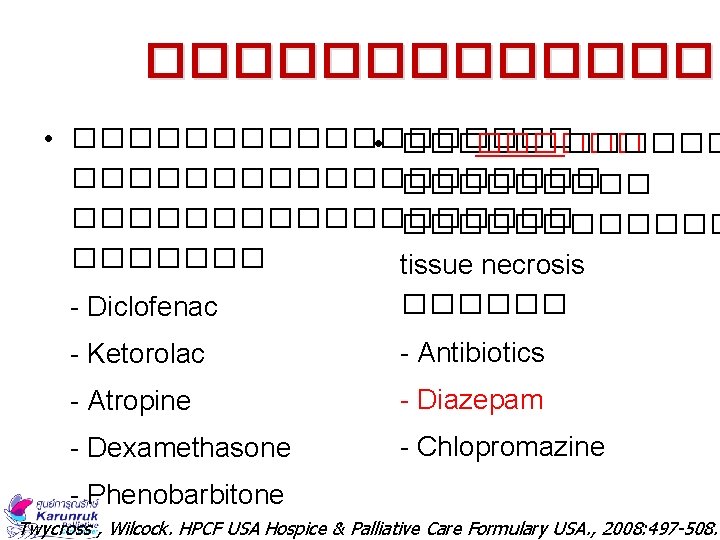
������� • ��������� • ������������������������ tissue necrosis - Diclofenac ������ - Ketorolac - Antibiotics - Atropine - Diazepam - Dexamethasone - Chlopromazine - Phenobarbitone Twycross , Wilcock. HPCF USA Hospice & Palliative Care Formulary USA. , 2008: 497 -508.
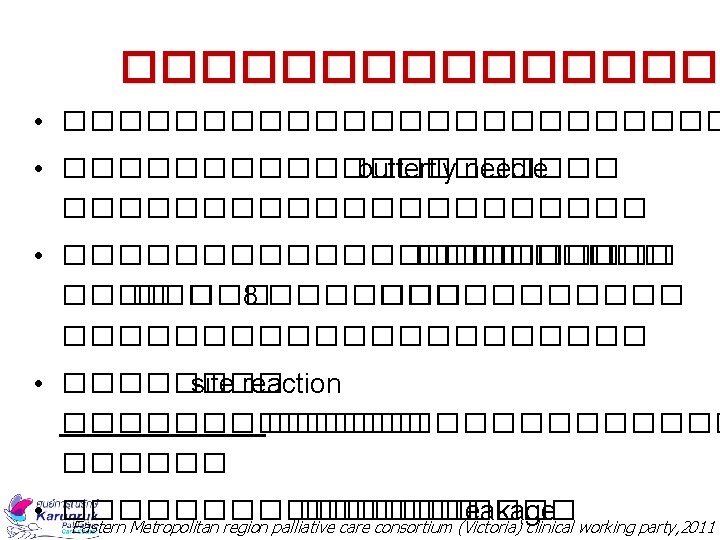



Equipment Supplied • • • Syringe driver Syringe disposable ���� 20 ml Scalp vein needle no. 25 Extension tube Tegaderm ���� 70% ������������ 9 �������������� Sticker �������

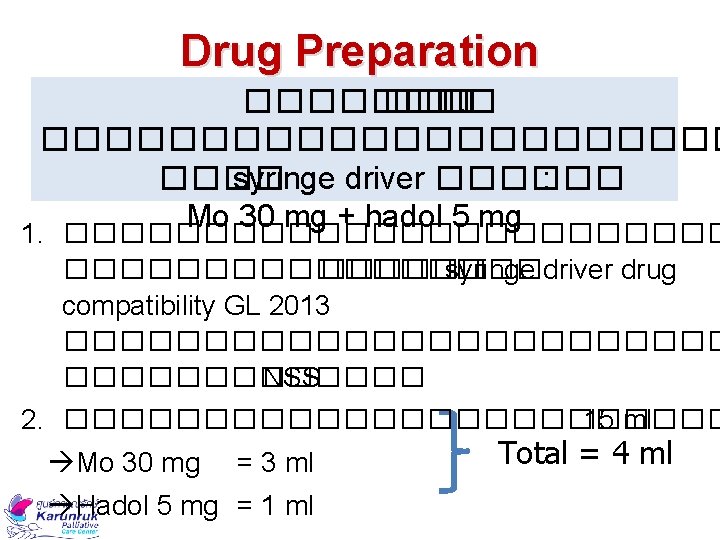
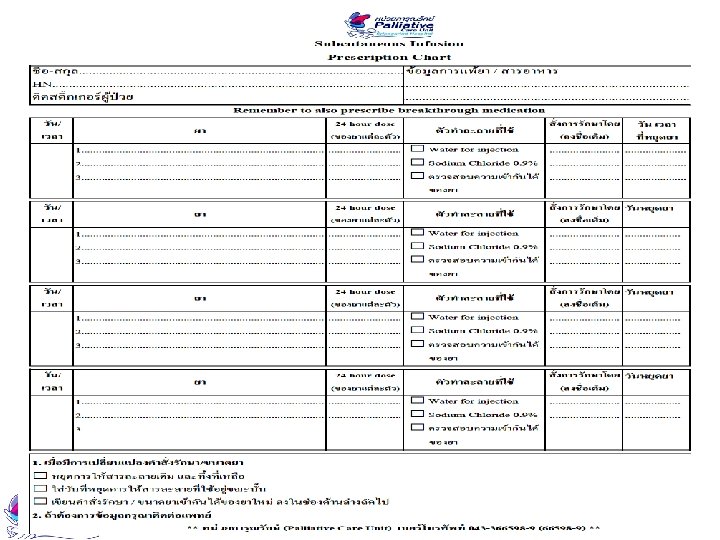
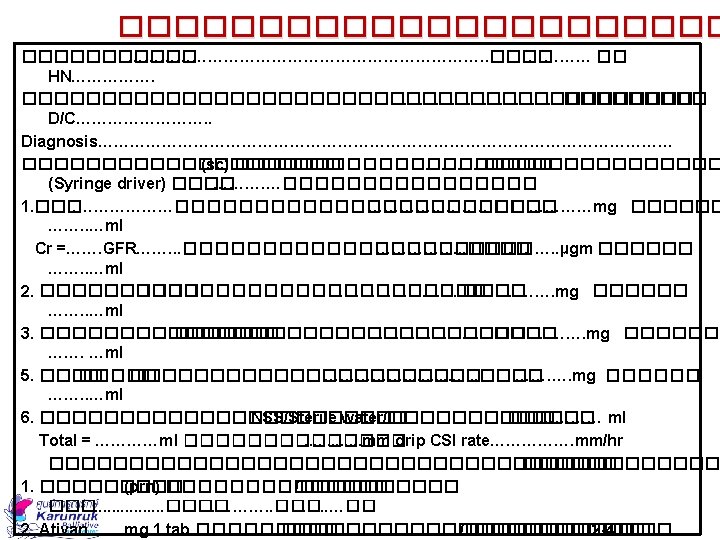
Drug Preparation ���� ��� �. ����������� syringe driver ������ : Mo 30 mg + hadol 5 mg 1. ������������ ���� syringe driver drug compatibility GL 2013 ������������ NSS 2. ������������ 15 ml Total = 4 ml Mo 30 mg = 3 ml Hadol 5 mg = 1 ml
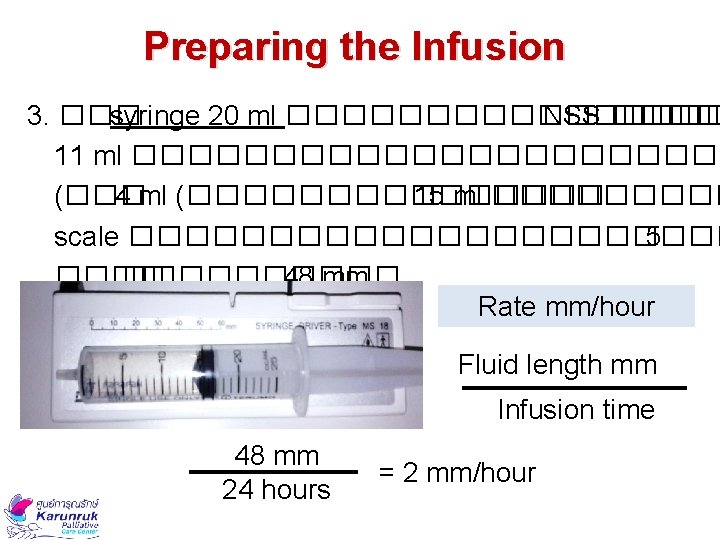
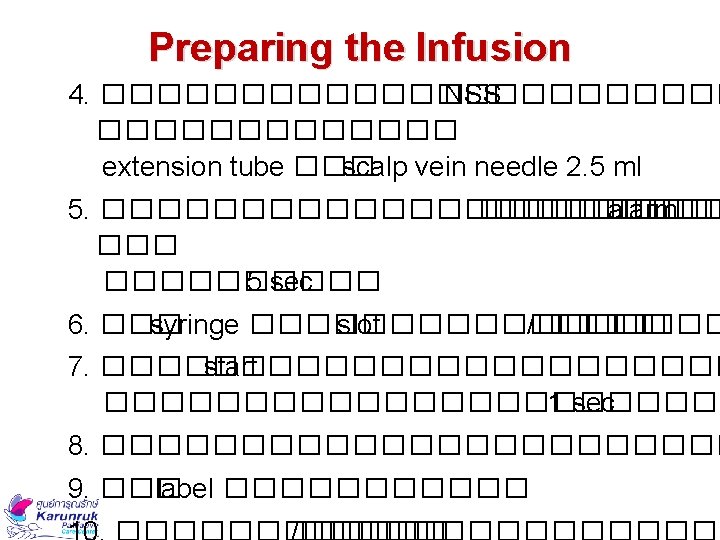
Preparing the Infusion 3. ��� syringe 20 ml �������� NSS ���� 11 ml ����������� (��� 4 ml (�������� 15 ml ����� scale ����������� 5 ���������� 48 mm Rate mm/hour Fluid length mm Infusion time 48 mm 24 hours = 2 mm/hour
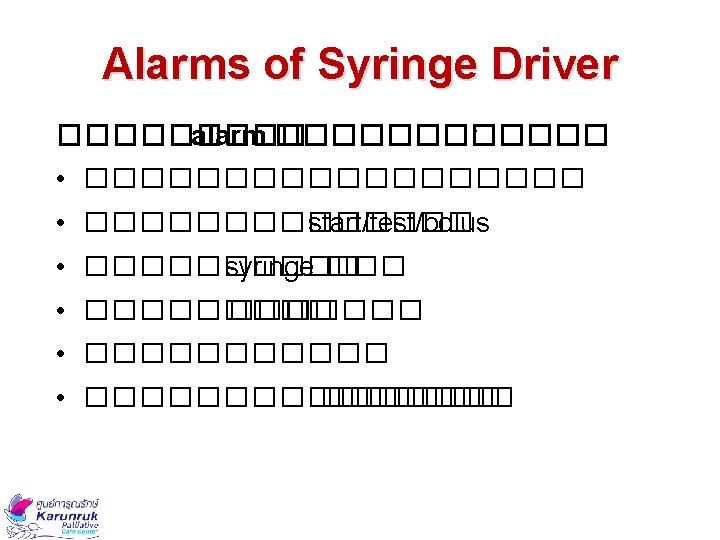

Frequently Asked Questions
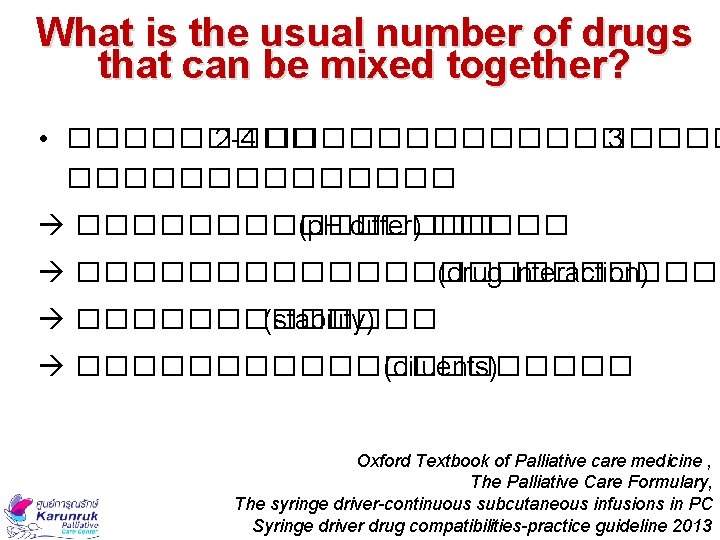
What is the usual number of drugs that can be mixed together? • ����� 2 -4 ��������� 3 �������� (p. H differ) ������������ (drug interaction) ������� (stability) ���������� (diluents) Oxford Textbook of Palliative care medicine , The Palliative Care Formulary, The syringe driver-continuous subcutaneous infusions in PC Syringe driver drug compatibilities-practice guideline 2013

Compatibility and Stability of Drugs • �������� stability ���� p. H ������������ 24 ���������������� • Instability ���� incompatibility ������������������������ • ����������� incompatibility ��������� Eastern Metropolitan region palliative care consortium (Victoria) clinical working party. Syringe driver drug compatibilities-practice guideline 2013

Preferred Diluent ? • ����� Syringe driver drug compatibilities practice guidelines 2013 • NSS ��� SWI ������������������ (stability) �� site reaction • �������� 2 -3 ������ syringe �������������� NSS ��� SWI- NSS ������������ Eastern Metropolitan region palliative care consortium (Victoria) clinical working party. Syringe driver drug compatibilities-practice guideline 2013
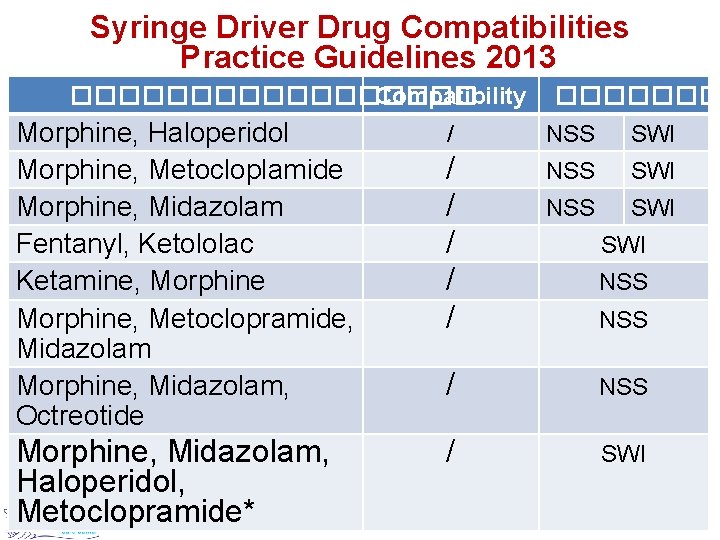
Syringe Driver Drug Compatibilities Practice Guidelines 2013 ��������� Compatibility Morphine, Haloperidol Morphine, Metocloplamide Morphine, Midazolam Fentanyl, Ketololac Ketamine, Morphine, Metoclopramide, Midazolam Morphine, Midazolam, Octreotide Morphine, Midazolam, Haloperidol, Metoclopramide* ������� / NSS SWI / / / NSS SWI SWI NSS / SWI

Reference • Dickman A, Littlewood C, & Varga J. The syringe driver: continuous subcutaneous infusion in palliative care. New York: Oxford University Press. • The Palliative Care Formulary • Eastern Metropolitan region palliative care consortium (Victoria) clinical working party. Syringe driver drug compatibilities-practice guideline 2013, Australia. • Greater Glasgow and Clyde. Guidelines for the use of subcutaneous medications in palliative care for adultsprimary care and hospices. NHS Greater Glasgow primary care palliative care team.
- Slides: 40