BASIC ORGANIC CHEMISTRY BY ASST PROF NANDAKISHOR PATIL

BASIC ORGANIC CHEMISTRY • BY • ASST. PROF. NANDAKISHOR PATIL Copyright Mc. Graw-Hill 2009 1
![10. 1 Why Carbon Is Different • Electron configuration: [He]2 s 22 p 2 10. 1 Why Carbon Is Different • Electron configuration: [He]2 s 22 p 2](http://slidetodoc.com/presentation_image_h2/2b6b9200cc5413f7593f1038cc1a6e2e/image-2.jpg)
10. 1 Why Carbon Is Different • Electron configuration: [He]2 s 22 p 2 effectively prohibits ion formation • Small atomic radius gives rise to short, strong C C bonds and stable compounds • Hybridized atoms (sp- and sp 2 -) can form strong p bonds with unhybridzed p orbitals • Catenation to form chains and rings containing single, double and triple bonds. Copyright Mc. Graw-Hill 2009 2
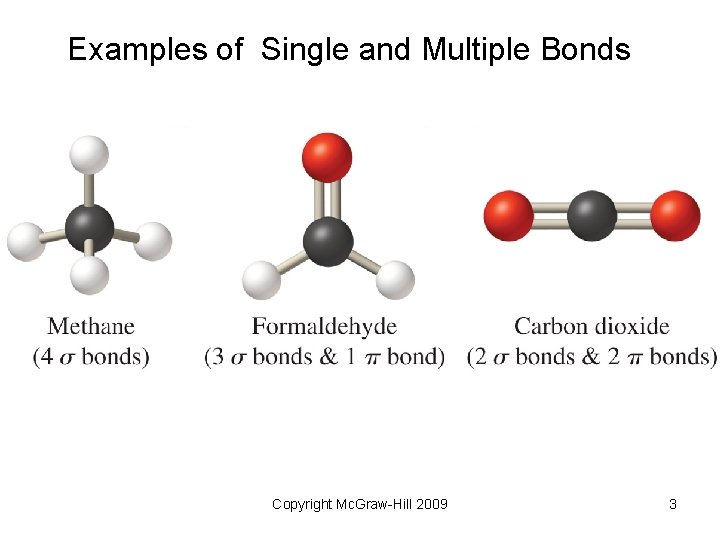
Examples of Single and Multiple Bonds Copyright Mc. Graw-Hill 2009 3
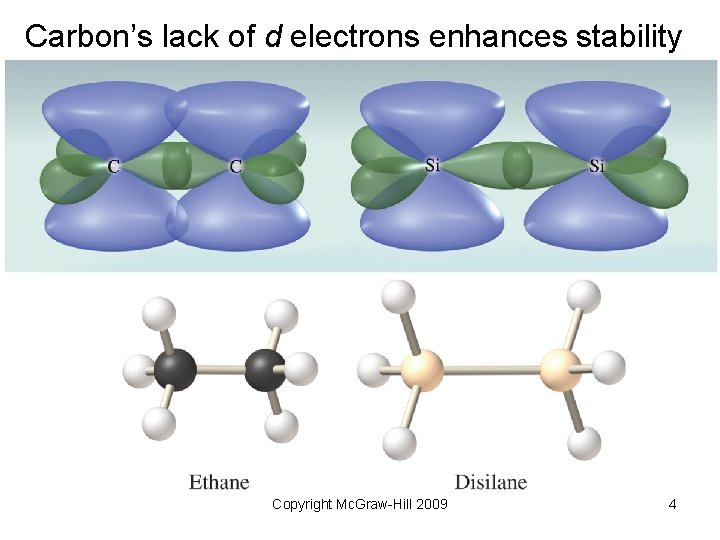
Carbon’s lack of d electrons enhances stability Copyright Mc. Graw-Hill 2009 4
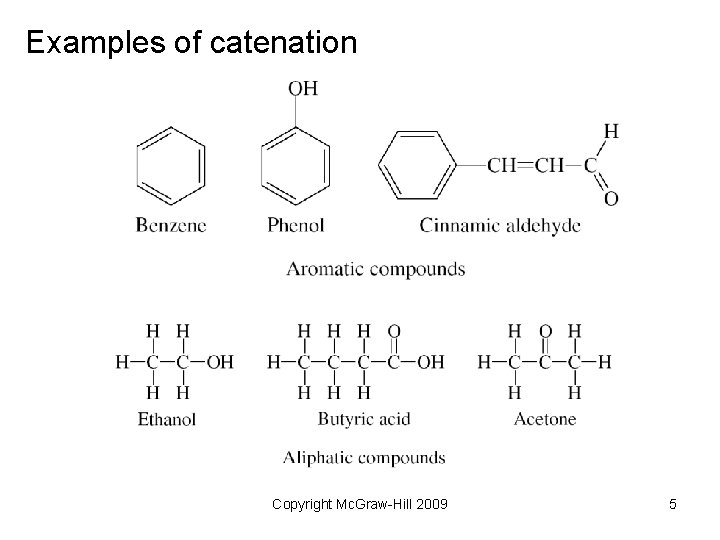
Examples of catenation Copyright Mc. Graw-Hill 2009 5
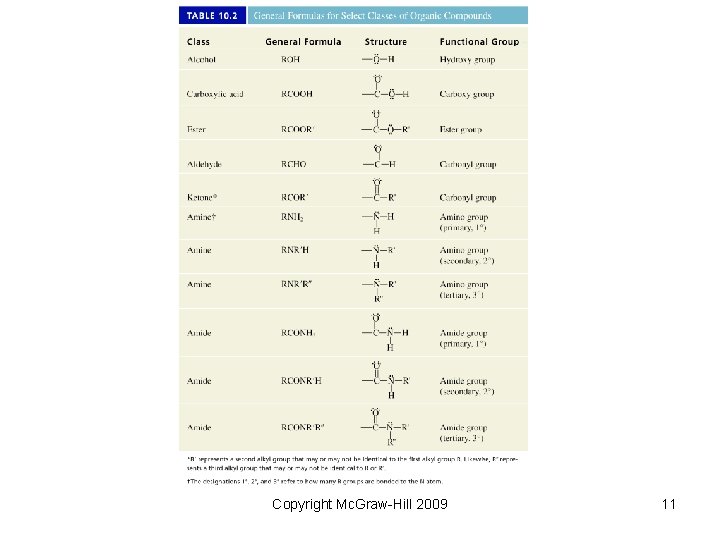
10. 2 Classes of Organic Compounds The seemingly limitless variety of organic compounds results from: • Carbon’s ability to form chains by bonding to itself • Presence of elements other than carbon and hydrogen • Functional groups – a group of atoms that determines many of a molecule’s properties • Multiple bonds Copyright Mc. Graw-Hill 2009 6
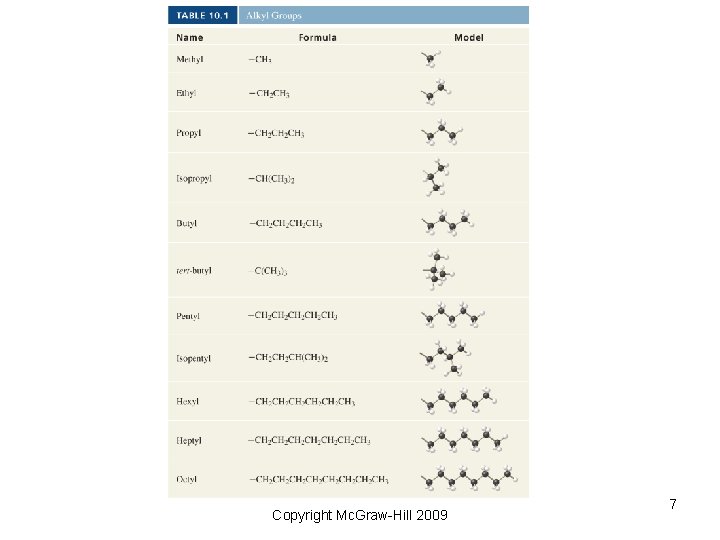
Copyright Mc. Graw-Hill 2009 7
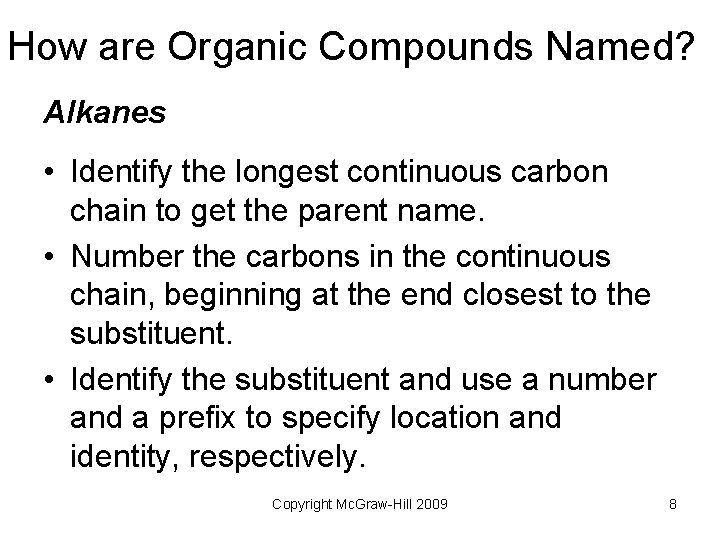
How are Organic Compounds Named? Alkanes • Identify the longest continuous carbon chain to get the parent name. • Number the carbons in the continuous chain, beginning at the end closest to the substituent. • Identify the substituent and use a number and a prefix to specify location and identity, respectively. Copyright Mc. Graw-Hill 2009 8
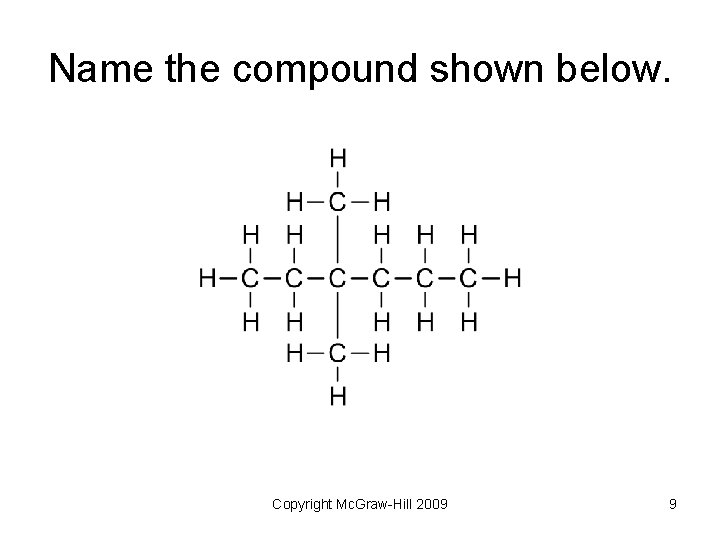
Name the compound shown below. Copyright Mc. Graw-Hill 2009 9
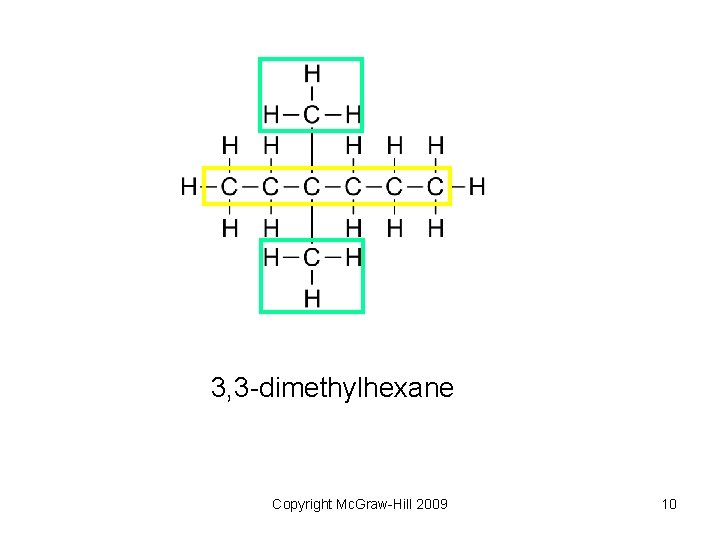
3, 3 -dimethylhexane Copyright Mc. Graw-Hill 2009 10
Copyright Mc. Graw-Hill 2009 11
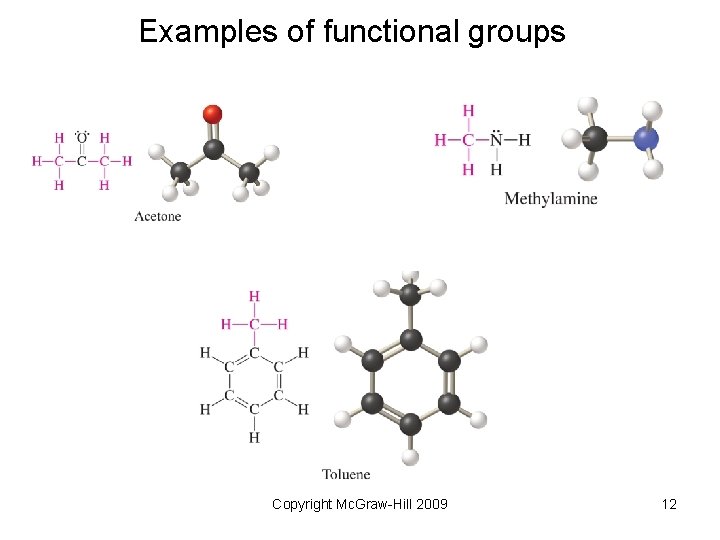
Examples of functional groups Copyright Mc. Graw-Hill 2009 12
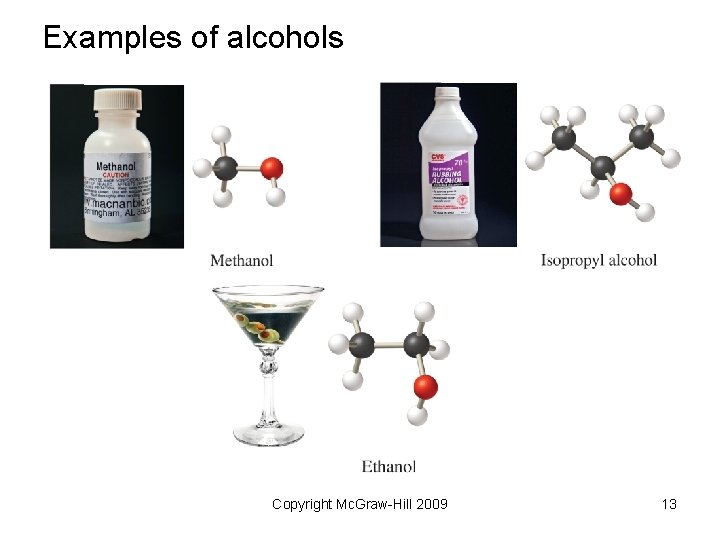
Examples of alcohols Copyright Mc. Graw-Hill 2009 13
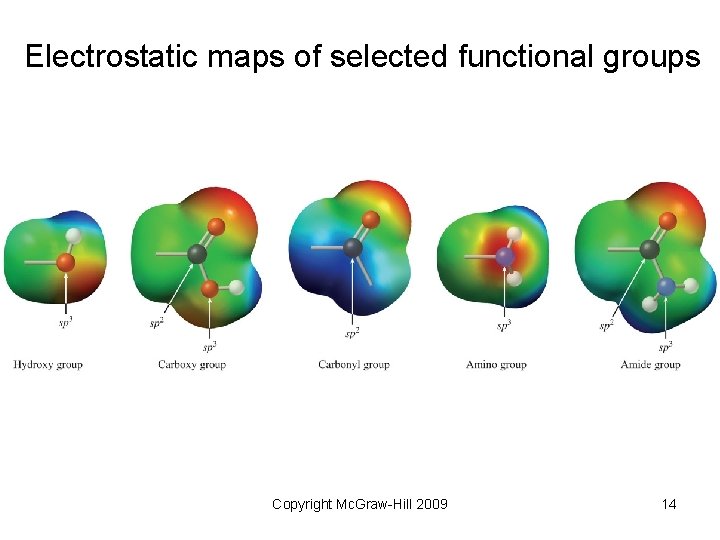
Electrostatic maps of selected functional groups Copyright Mc. Graw-Hill 2009 14

Naming Specific Functional Groups Alcohols • Identify the longest chain that includes the –OH group. • Change the –e ending to -ol. • Number to give the –OH the lowest number. • When the chain also contains an alkyl substituent, give the –OH the lowest number. Copyright Mc. Graw-Hill 2009 15
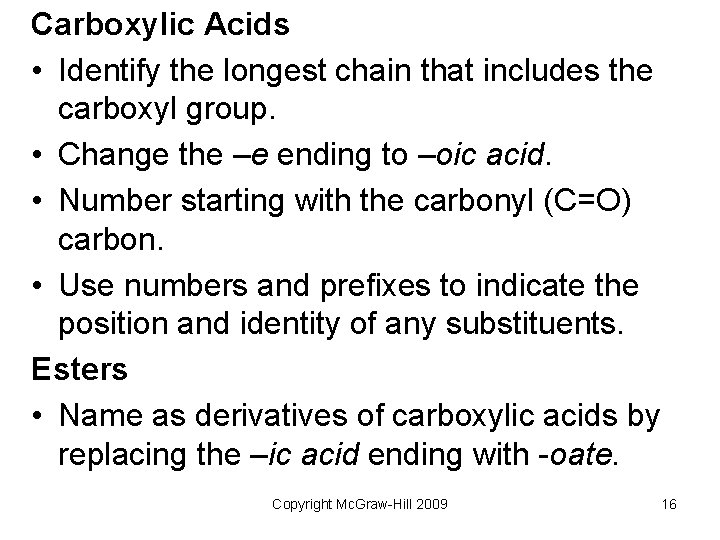
Carboxylic Acids • Identify the longest chain that includes the carboxyl group. • Change the –e ending to –oic acid. • Number starting with the carbonyl (C=O) carbon. • Use numbers and prefixes to indicate the position and identity of any substituents. Esters • Name as derivatives of carboxylic acids by replacing the –ic acid ending with -oate. Copyright Mc. Graw-Hill 2009 16
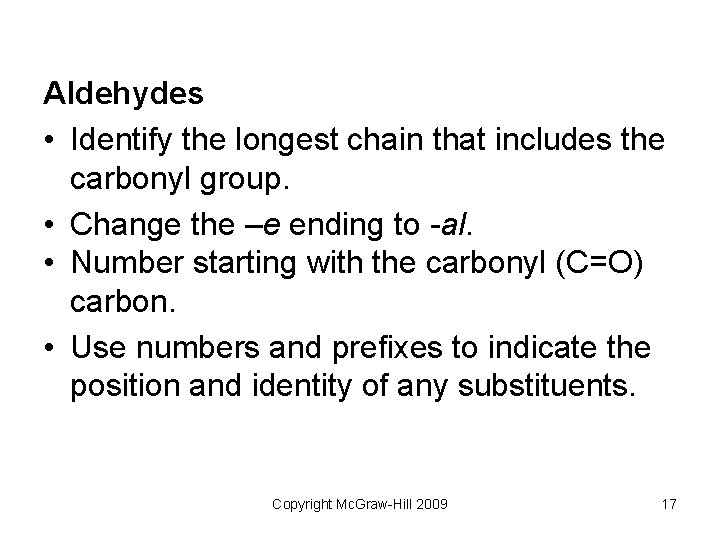
Aldehydes • Identify the longest chain that includes the carbonyl group. • Change the –e ending to -al. • Number starting with the carbonyl (C=O) carbon. • Use numbers and prefixes to indicate the position and identity of any substituents. Copyright Mc. Graw-Hill 2009 17

Ketones • Identify the longest chain that includes the carbonyl group. • Change the –e ending to -one. • Number to give the carbonyl group the lowest possible number. • Use numbers and prefixes to indicate the position and identity of any substituents. Copyright Mc. Graw-Hill 2009 18

Primary Amines • Identify the longest chain that includes the – NH 2 group. • Change the –e ending to -amine. • Number starting with the carbon to which the –NH 2 group is bonded. • Use numbers and prefixes to indicate the position and identity of any substituents. Primary Amides • Can be named as derivatives of carboxylic acids. • Or, by replacing the –e ending with –amide. Copyright Mc. Graw-Hill 2009 19

Compounds with More Than One Substituent • Prefixes of di, tri, tetra, penta and so forth are used to denote the number of substituents. • Substituent names are alphabetized. • Numbers are used to indicate position of the alphabetized substituents. • Prefixes are not used in alphabetization. Copyright Mc. Graw-Hill 2009 20
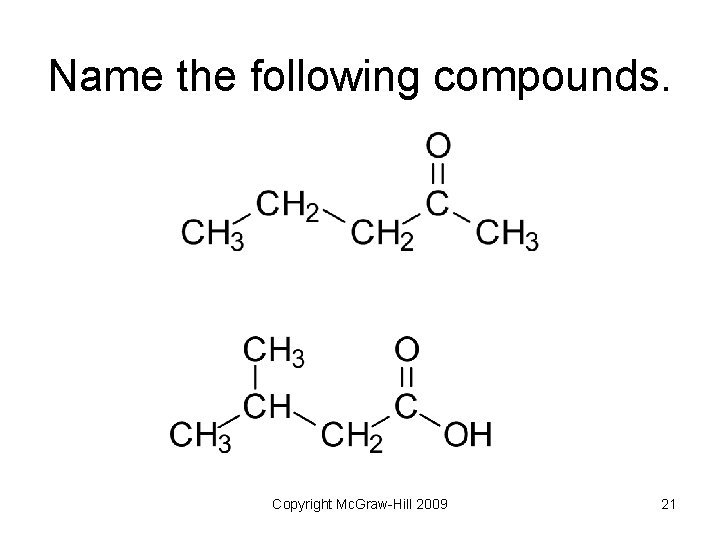
Name the following compounds. Copyright Mc. Graw-Hill 2009 21
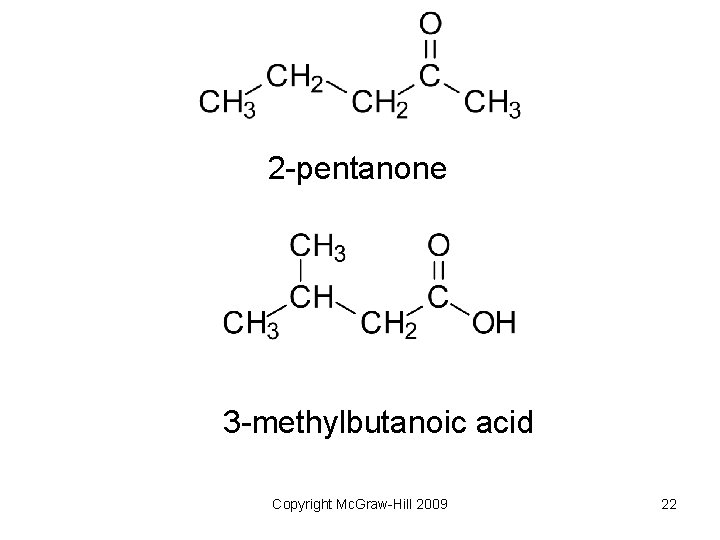
2 -pentanone 3 -methylbutanoic acid Copyright Mc. Graw-Hill 2009 22
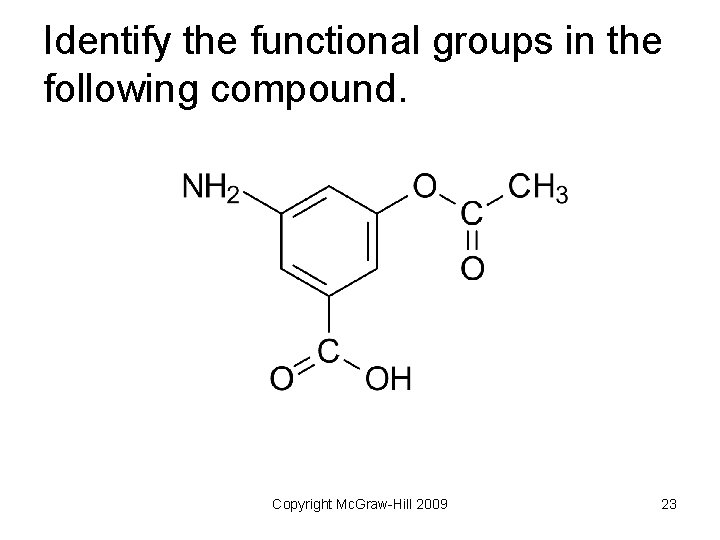
Identify the functional groups in the following compound. Copyright Mc. Graw-Hill 2009 23
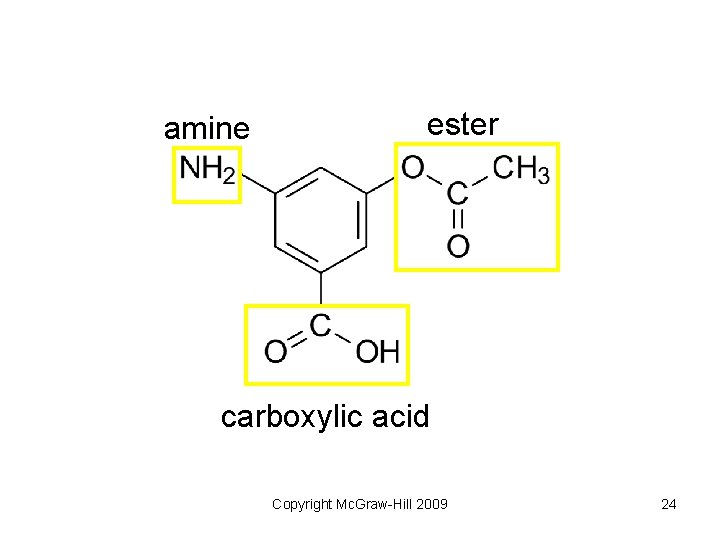
amine ester carboxylic acid Copyright Mc. Graw-Hill 2009 24
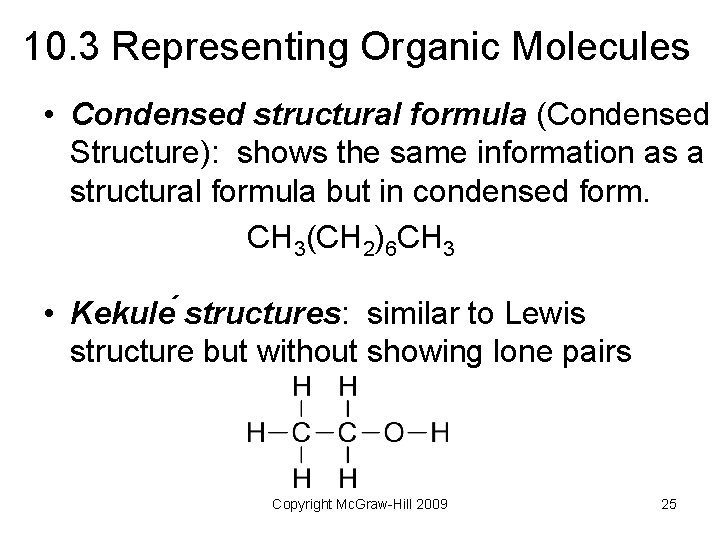
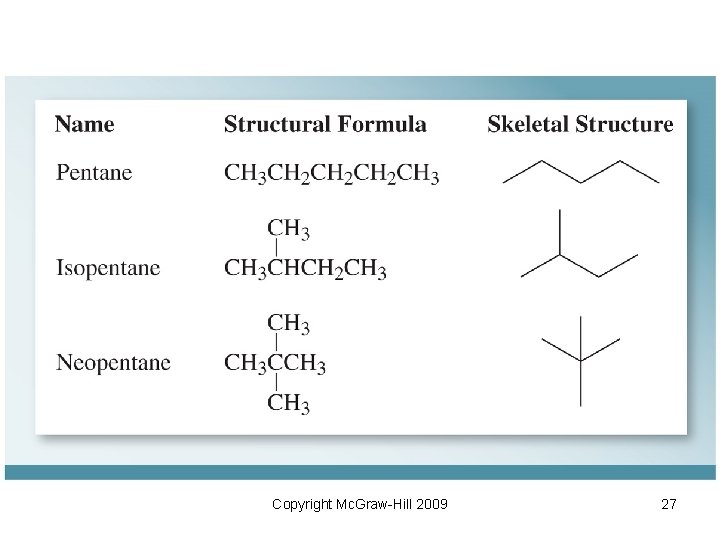
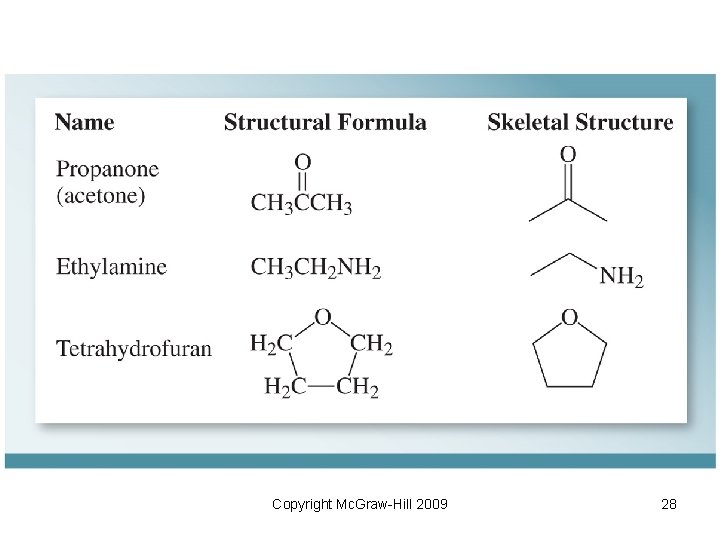
10. 3 Representing Organic Molecules • Condensed structural formula (Condensed Structure): shows the same information as a structural formula but in condensed form. CH 3(CH 2)6 CH 3 • Kekule structures: similar to Lewis structure but without showing lone pairs Copyright Mc. Graw-Hill 2009 25
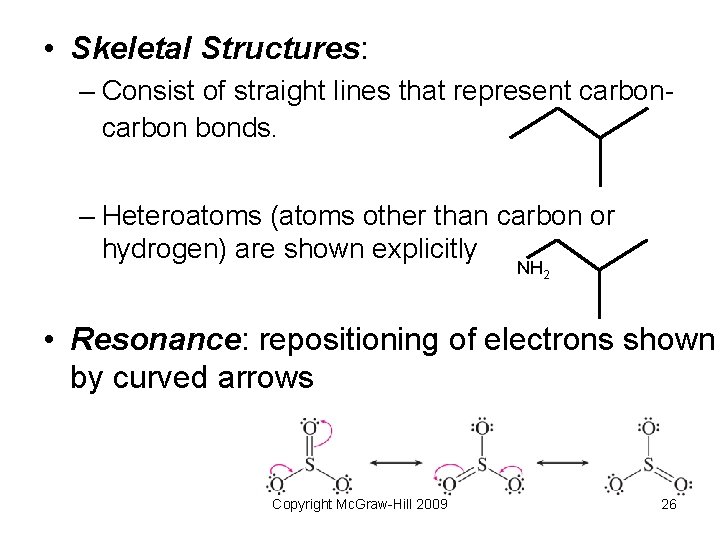
• Skeletal Structures: – Consist of straight lines that represent carbon bonds. – Heteroatoms (atoms other than carbon or hydrogen) are shown explicitly NH 2 • Resonance: repositioning of electrons shown by curved arrows Copyright Mc. Graw-Hill 2009 26
Copyright Mc. Graw-Hill 2009 27
Copyright Mc. Graw-Hill 2009 28
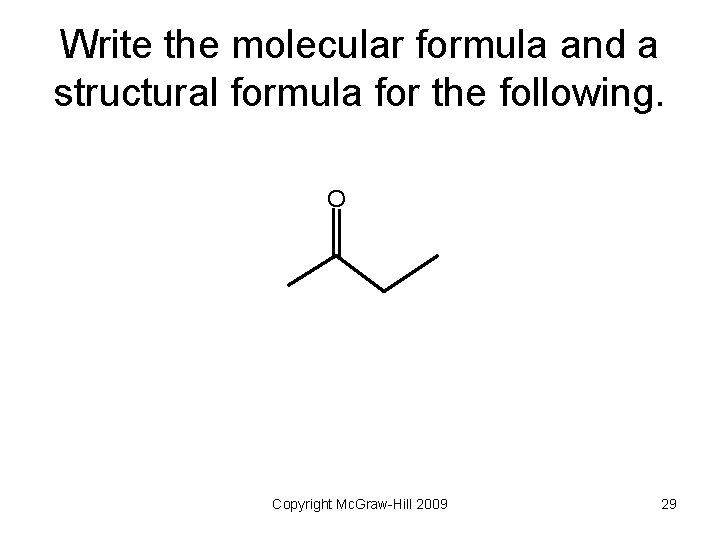
Write the molecular formula and a structural formula for the following. O Copyright Mc. Graw-Hill 2009 29
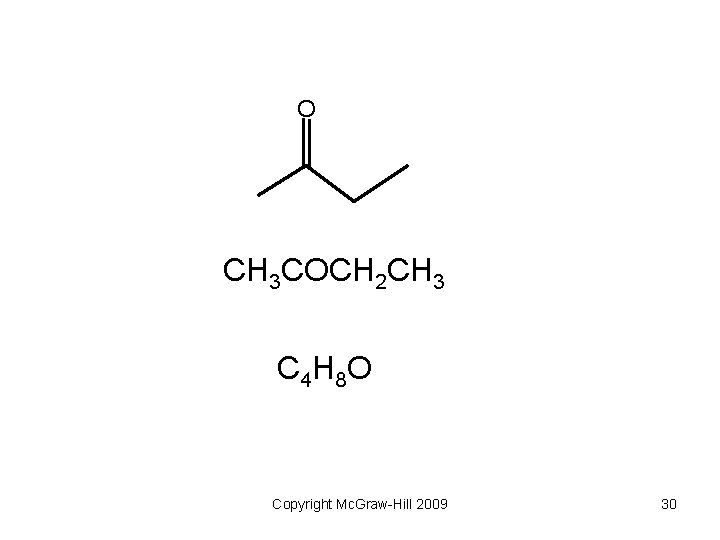
O CH 3 COCH 2 CH 3 C 4 H 8 O Copyright Mc. Graw-Hill 2009 30
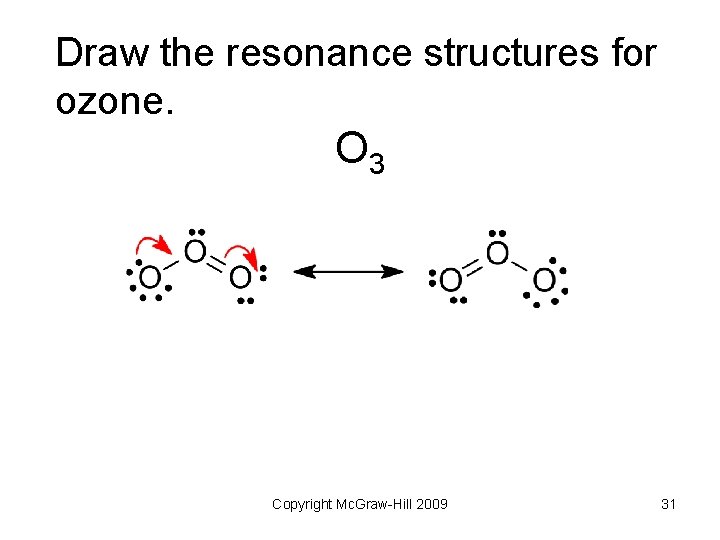
Draw the resonance structures for ozone. O 3 Copyright Mc. Graw-Hill 2009 31
10. 4 Isomerism • Constitutional (structural) isomerism occurs when the same atoms can be connected in two or more different ways. • Stereisomerism occurs when atoms are bonded in identical ways but differ in the orientation of those bonds in space. – Geometrical Isomers – Optical Isomers Copyright Mc. Graw-Hill 2009 32
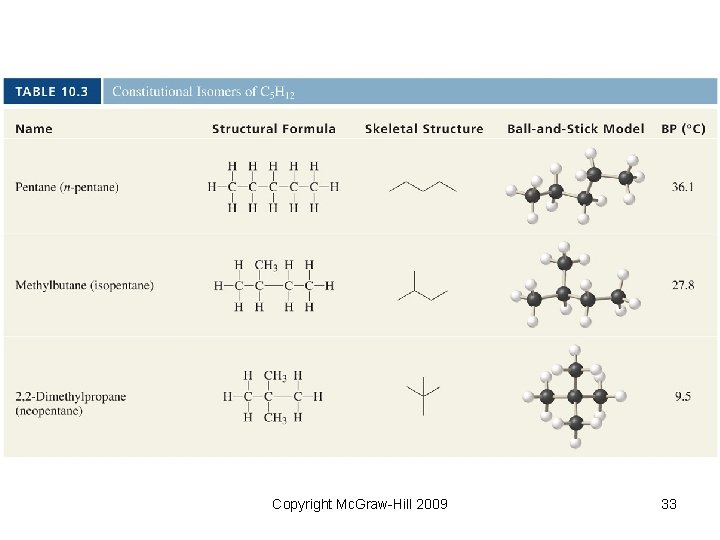
Copyright Mc. Graw-Hill 2009 33
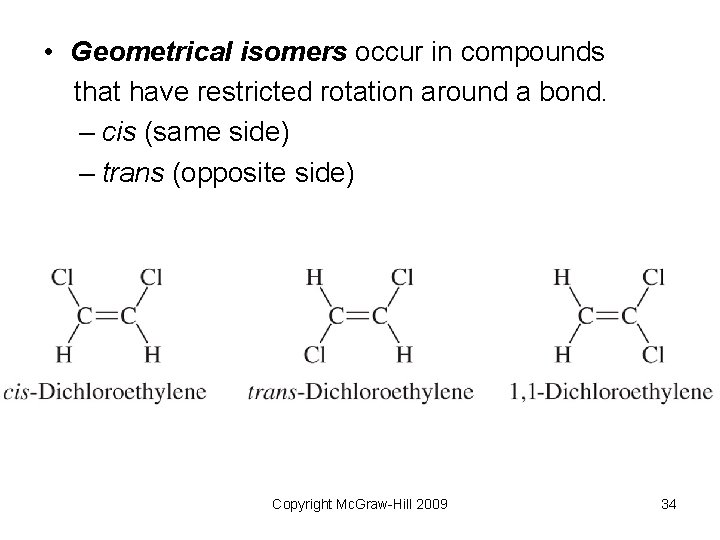
• Geometrical isomers occur in compounds that have restricted rotation around a bond. – cis (same side) – trans (opposite side) Copyright Mc. Graw-Hill 2009 34
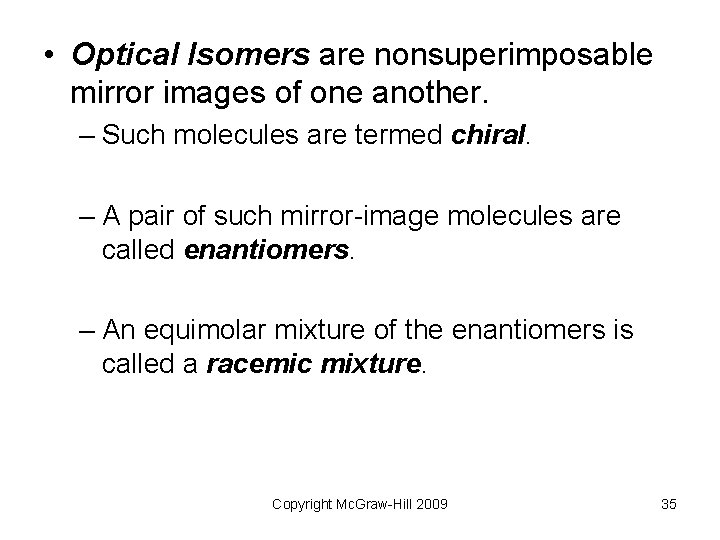
• Optical Isomers are nonsuperimposable mirror images of one another. – Such molecules are termed chiral. – A pair of such mirror-image molecules are called enantiomers. – An equimolar mixture of the enantiomers is called a racemic mixture. Copyright Mc. Graw-Hill 2009 35
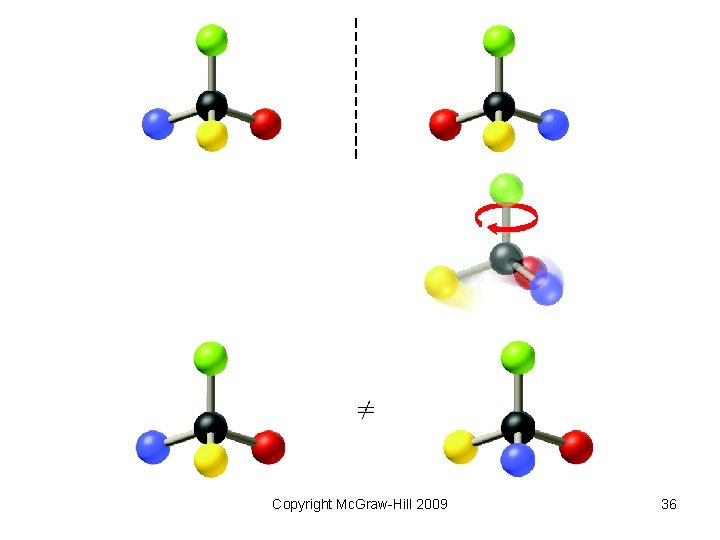
Copyright Mc. Graw-Hill 2009 36
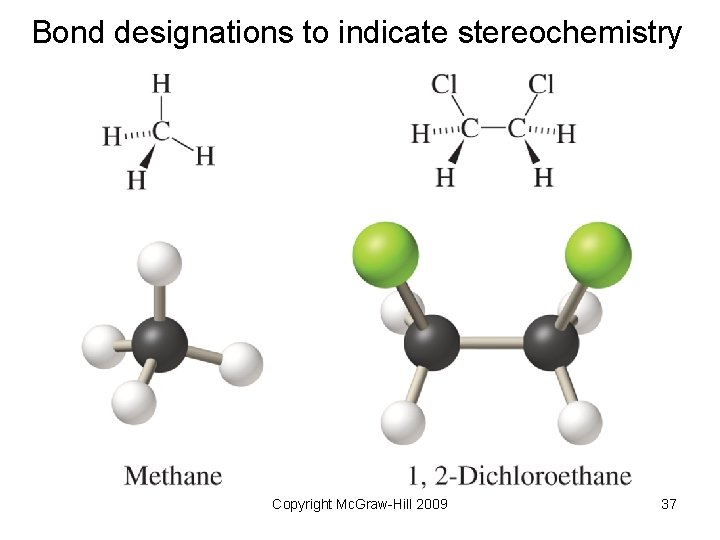
Bond designations to indicate stereochemistry Copyright Mc. Graw-Hill 2009 37
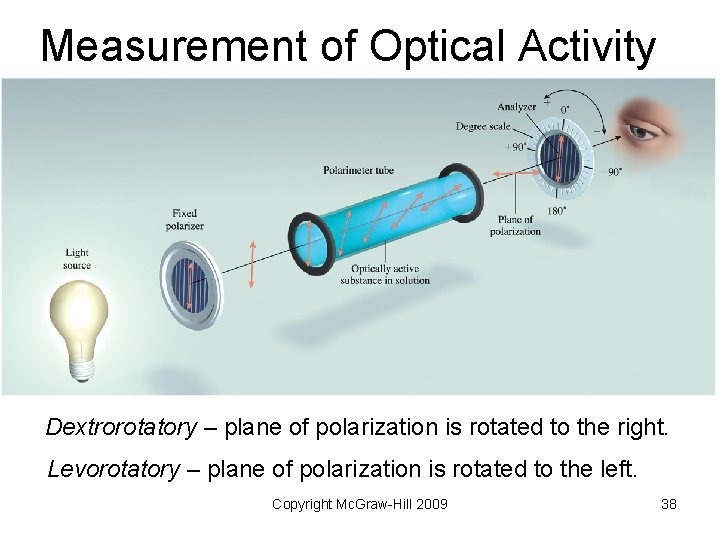
Measurement of Optical Activity Dextrorotatory – plane of polarization is rotated to the right. Levorotatory – plane of polarization is rotated to the left. Copyright Mc. Graw-Hill 2009 38
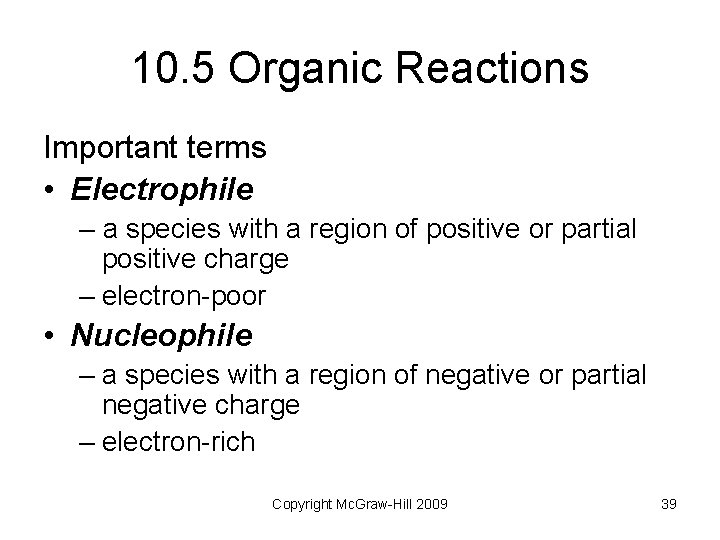
10. 5 Organic Reactions Important terms • Electrophile – a species with a region of positive or partial positive charge – electron-poor • Nucleophile – a species with a region of negative or partial negative charge – electron-rich Copyright Mc. Graw-Hill 2009 39
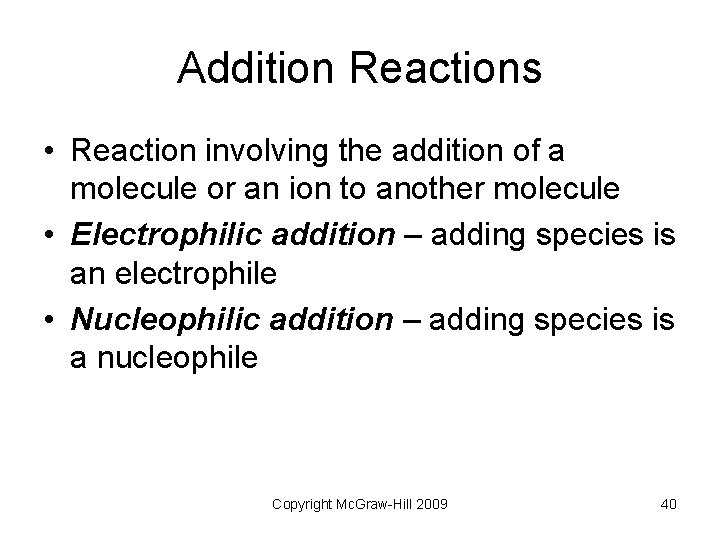
Addition Reactions • Reaction involving the addition of a molecule or an ion to another molecule • Electrophilic addition – adding species is an electrophile • Nucleophilic addition – adding species is a nucleophile Copyright Mc. Graw-Hill 2009 40
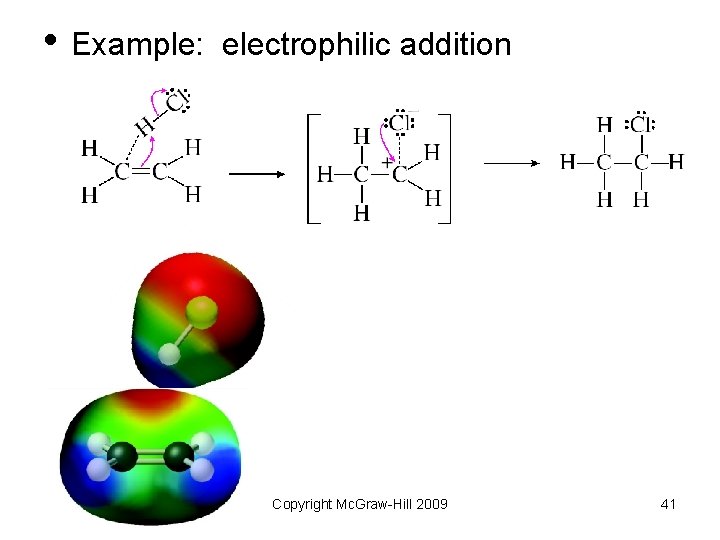
• Example: electrophilic addition Copyright Mc. Graw-Hill 2009 41
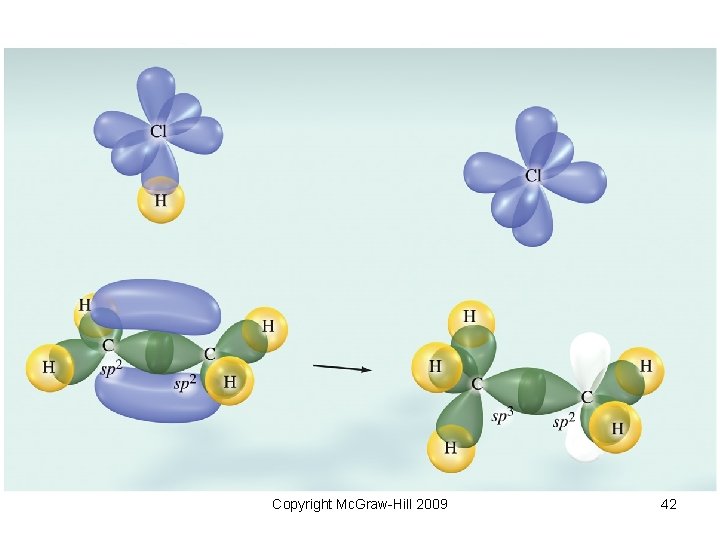
Text Figure 1038 Copyright Mc. Graw-Hill 2009 42
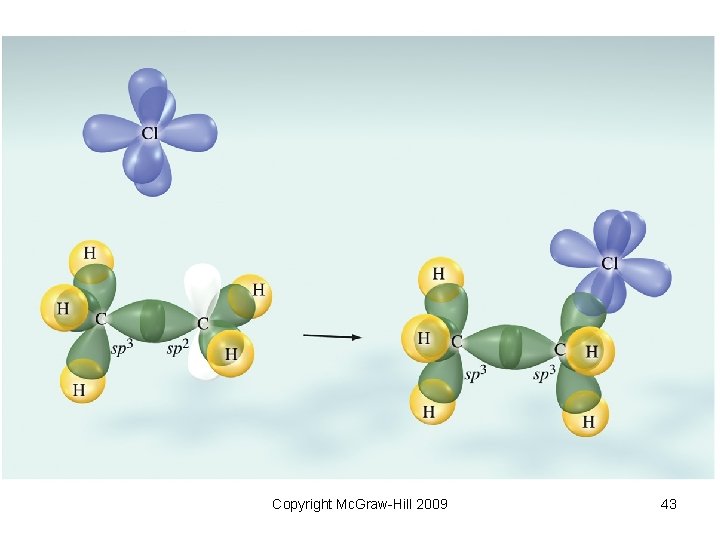
Copyright Mc. Graw-Hill 2009 43
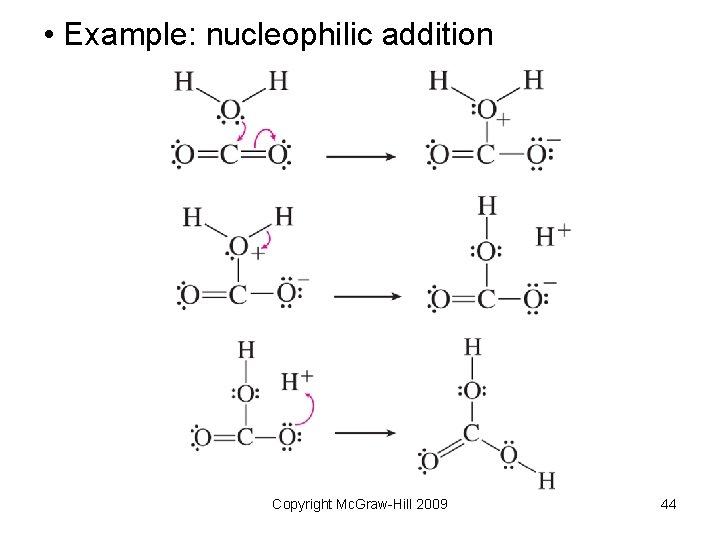
• Example: nucleophilic addition Copyright Mc. Graw-Hill 2009 44
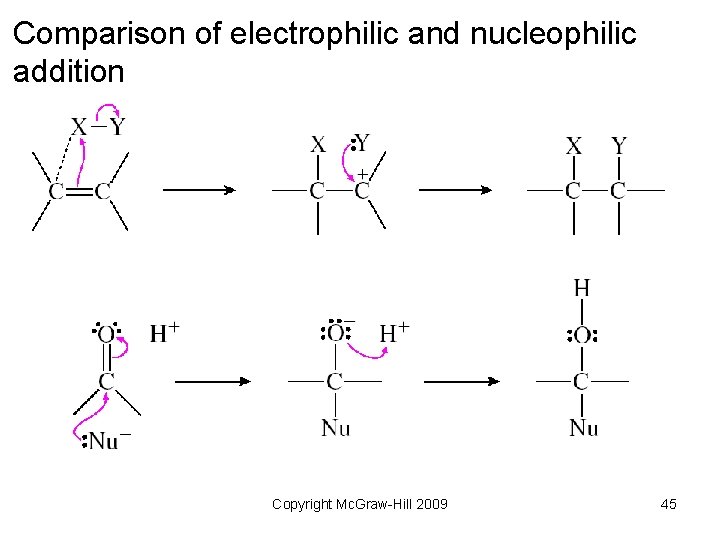
Comparison of electrophilic and nucleophilic addition Copyright Mc. Graw-Hill 2009 45
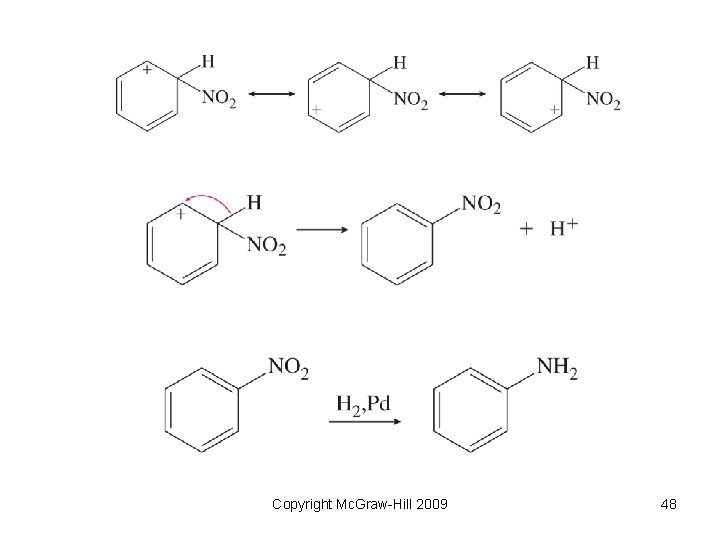
Substitution Reactions • Reaction when one group is replaced by another. • Electrophilic substitution – an electrophile attacks an aromatic molecule and replaces a hydrogen atom • Nucleophilic substitution – a nucleophile replaces another group on a carbon atom Copyright Mc. Graw-Hill 2009 46
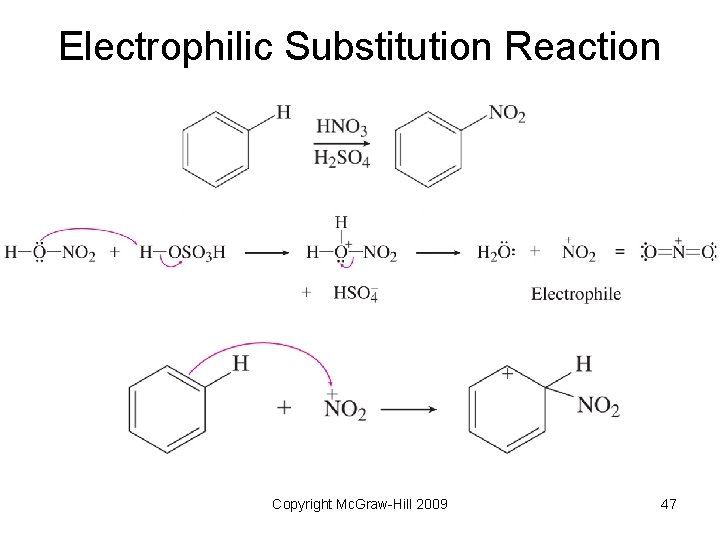
Electrophilic Substitution Reaction Copyright Mc. Graw-Hill 2009 47
Copyright Mc. Graw-Hill 2009 48
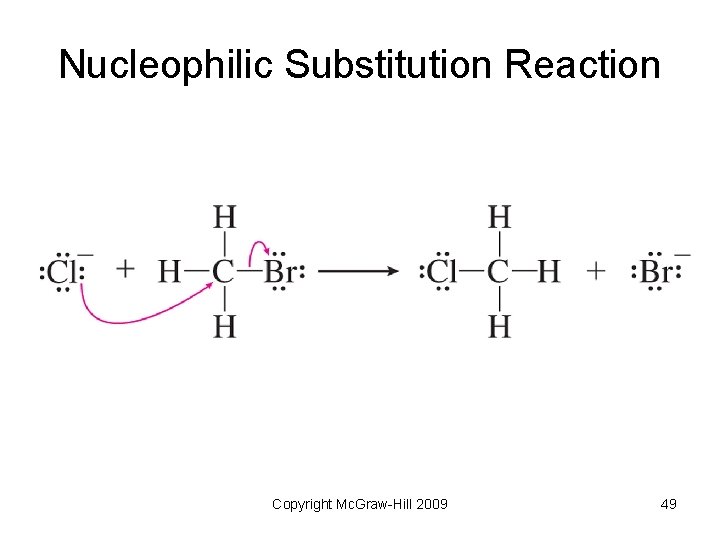
Nucleophilic Substitution Reaction Copyright Mc. Graw-Hill 2009 49
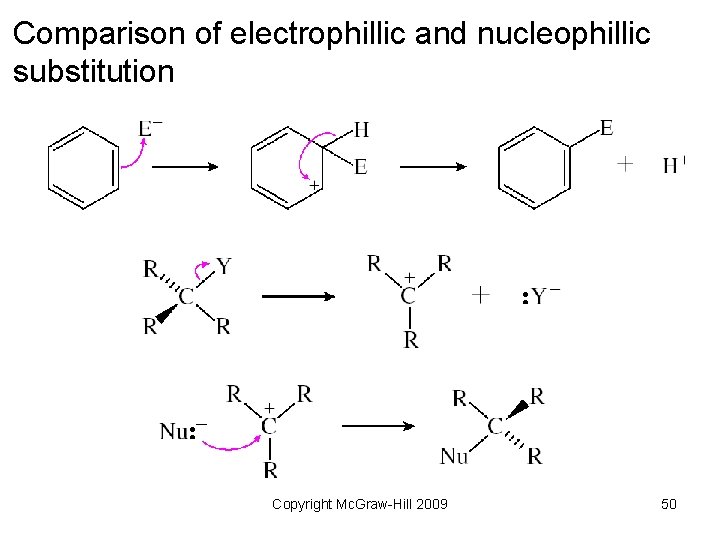
Comparison of electrophillic and nucleophillic substitution Copyright Mc. Graw-Hill 2009 50
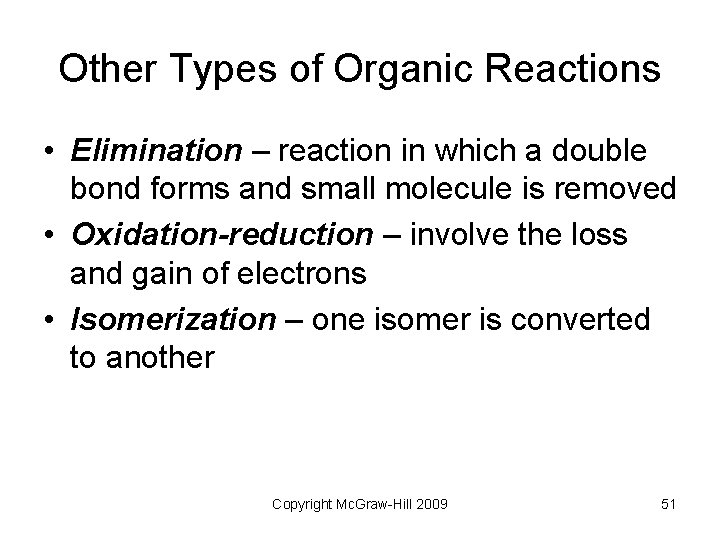
Other Types of Organic Reactions • Elimination – reaction in which a double bond forms and small molecule is removed • Oxidation-reduction – involve the loss and gain of electrons • Isomerization – one isomer is converted to another Copyright Mc. Graw-Hill 2009 51
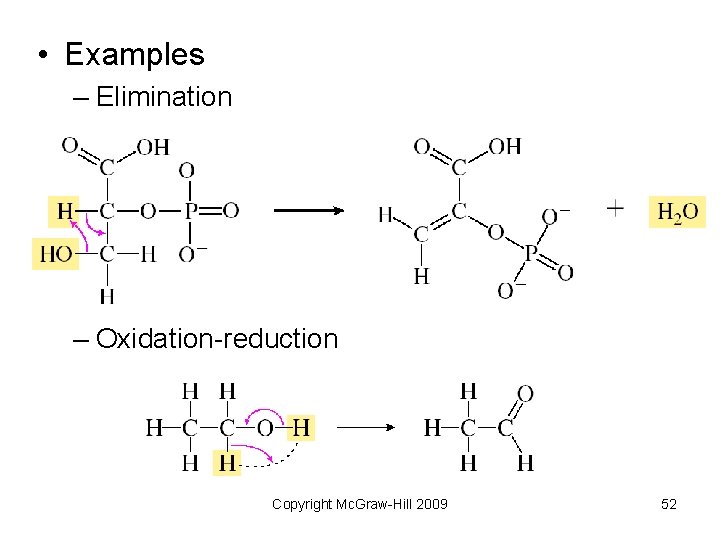
• Examples – Elimination – Oxidation-reduction Copyright Mc. Graw-Hill 2009 52
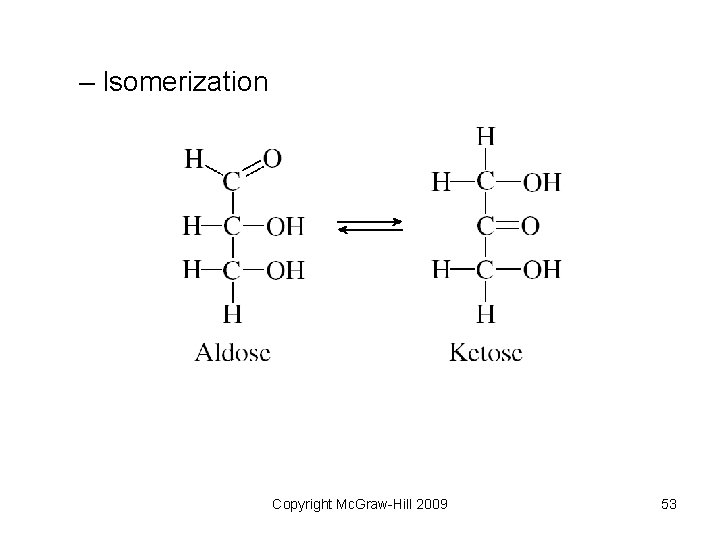
– Isomerization Copyright Mc. Graw-Hill 2009 53
Draw the mechanism for the nucleophilic addition of CN to CH 3 CHO. Copyright Mc. Graw-Hill 2009 54
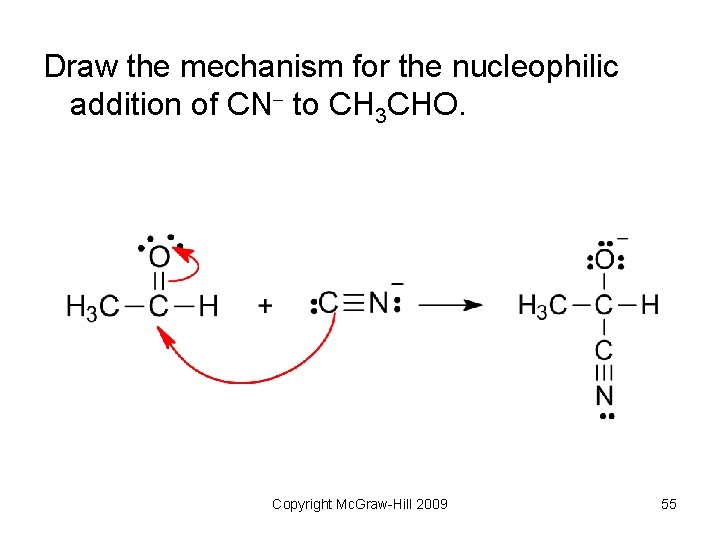
Draw the mechanism for the nucleophilic addition of CN to CH 3 CHO. Copyright Mc. Graw-Hill 2009 55
10. 6 Organic Polymers • Polymers – molecular compounds made up of many repeating units called monomers • Types – Addition polymers form when monomers join end to end – Condensation polymers form when two different functional groups combine in an elimination reaction • Often are copolymers which are made of two or more different monomers Copyright Mc. Graw-Hill 2009 56
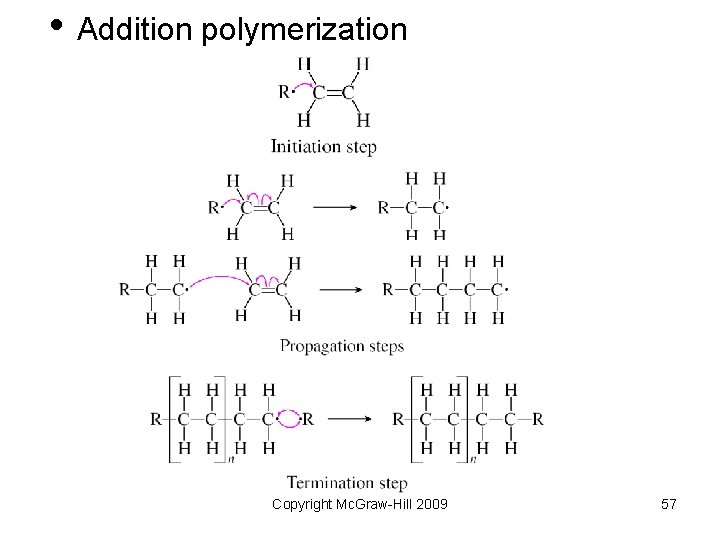
• Addition polymerization Copyright Mc. Graw-Hill 2009 57
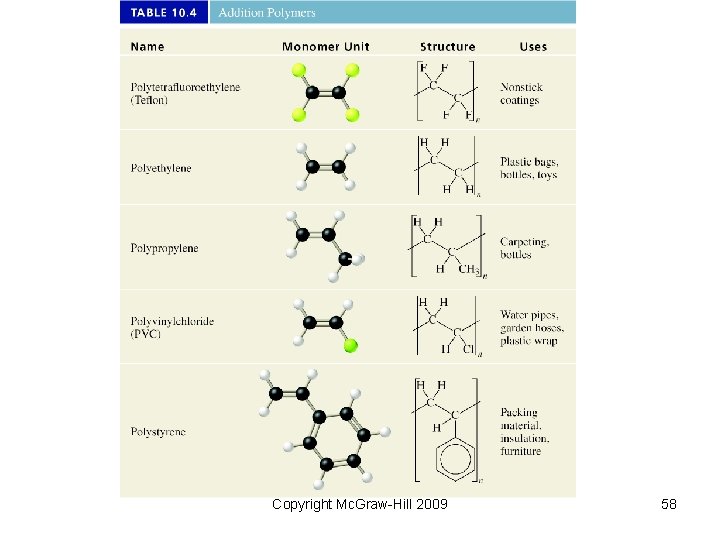
Copyright Mc. Graw-Hill 2009 58
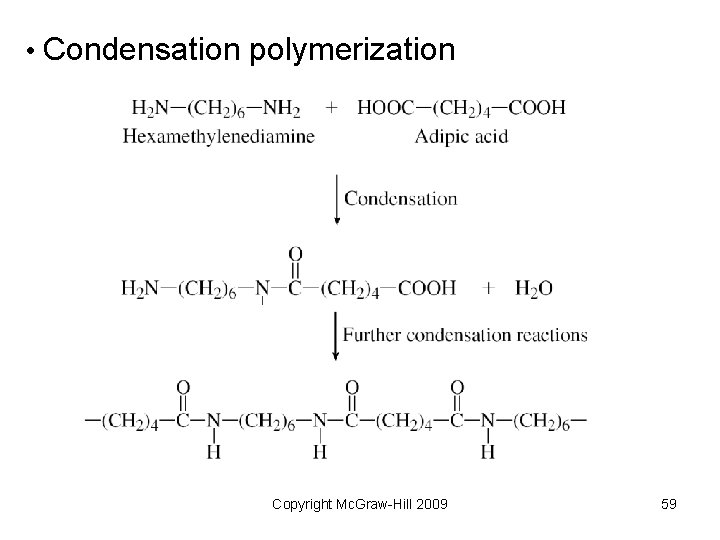
• Condensation polymerization Copyright Mc. Graw-Hill 2009 59
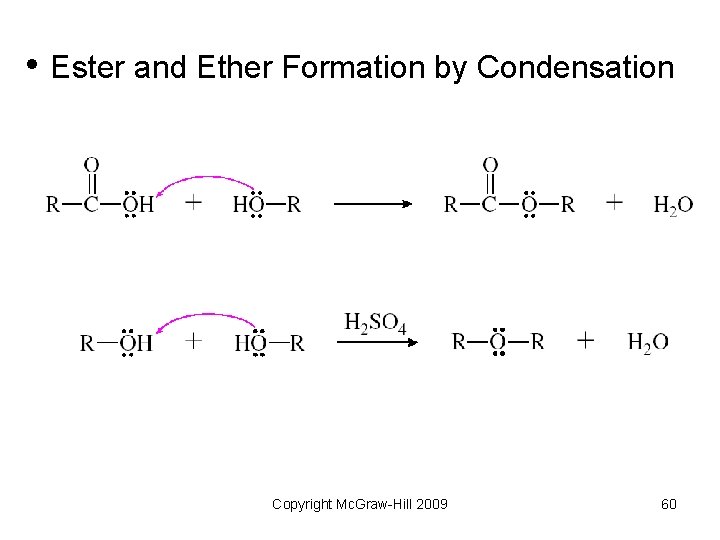
• Ester and Ether Formation by Condensation Copyright Mc. Graw-Hill 2009 60
Biological Polymers Naturally occurring polymers include • Proteins – polymers of amino acids • Polysaccharides – polymers of sugars • Nucleic acids – polymers of nucleotides – DNA (deoxyribonucleic acid) – RNA (ribonucleic acid) Copyright Mc. Graw-Hill 2009 61
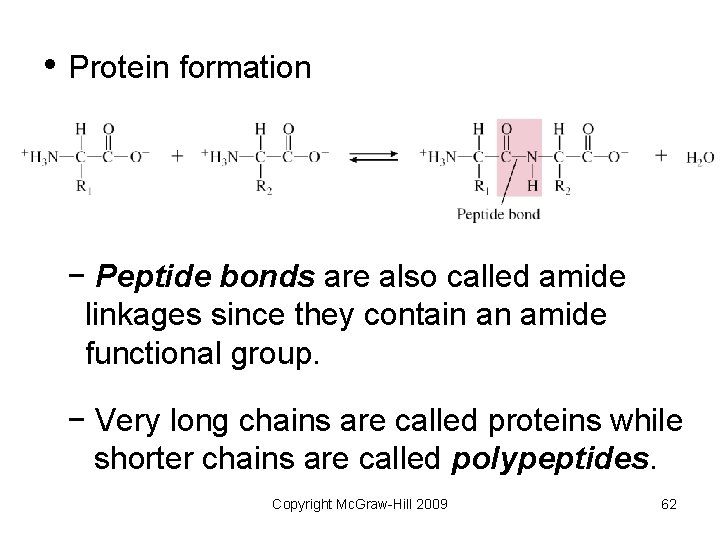
• Protein formation − Peptide bonds are also called amide linkages since they contain an amide functional group. − Very long chains are called proteins while shorter chains are called polypeptides. Copyright Mc. Graw-Hill 2009 62
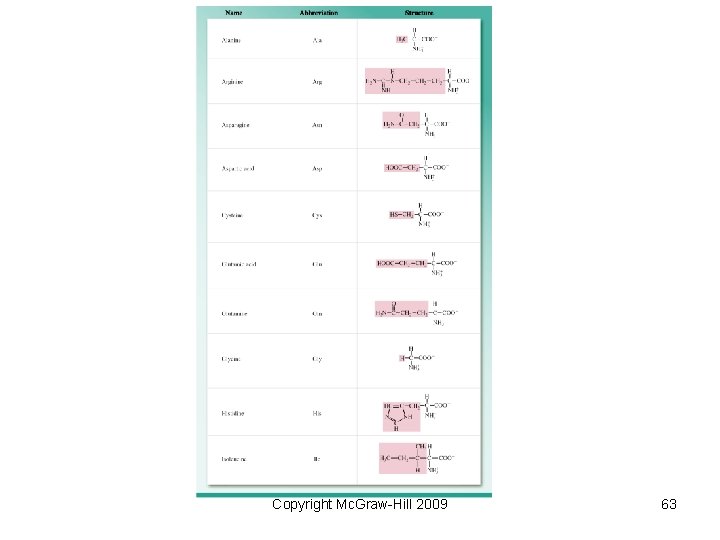
Copyright Mc. Graw-Hill 2009 63
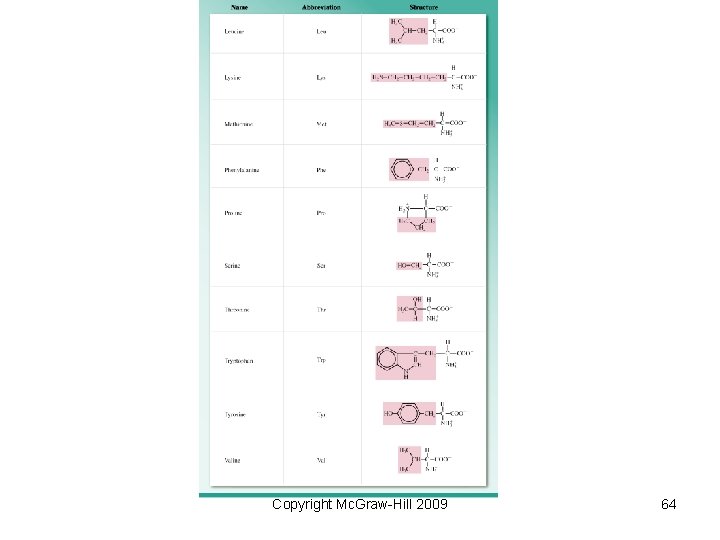
Copyright Mc. Graw-Hill 2009 64
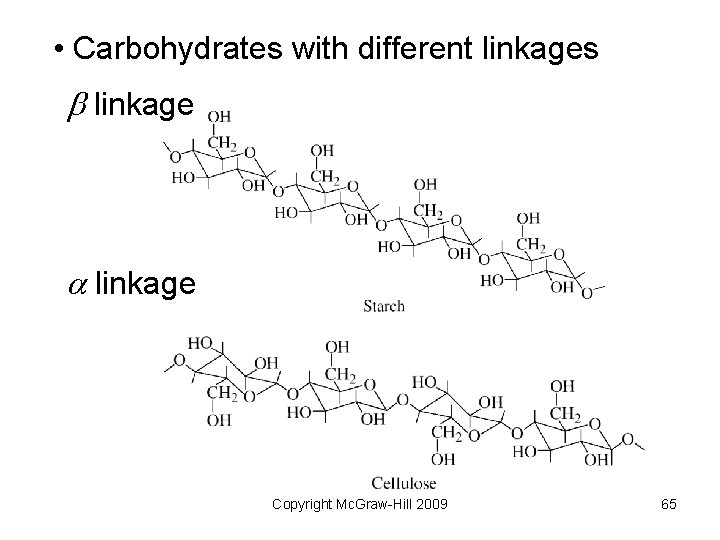
• Carbohydrates with different linkages b linkage a linkage Copyright Mc. Graw-Hill 2009 65
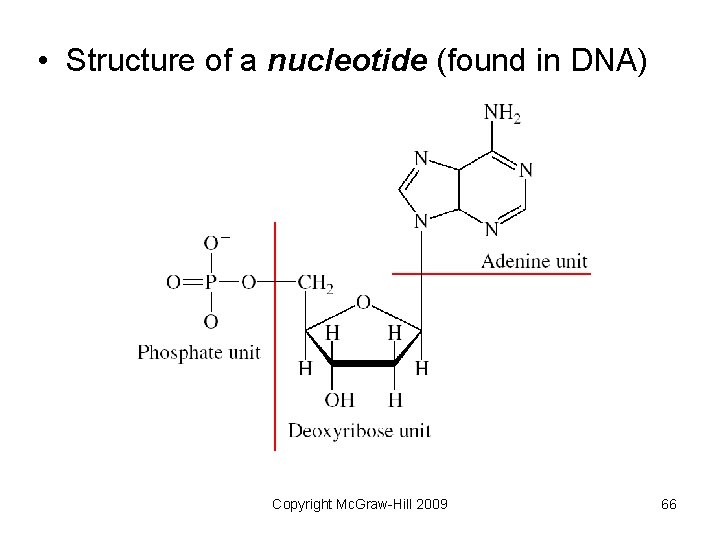
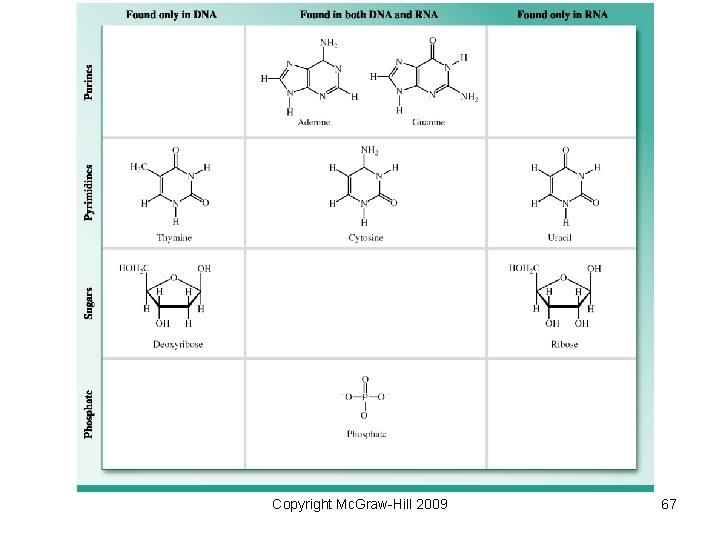
• Structure of a nucleotide (found in DNA) Copyright Mc. Graw-Hill 2009 66
Copyright Mc. Graw-Hill 2009 67
Key Points • • Unique features of carbon Classes of organic compounds Naming organic compounds Isomerism – Constitutional isomerism – Stereoisomerism • Geometrical isomers • Optical isomers Copyright Mc. Graw-Hill 2009 68
Key Points • Organic reactions – Addition reactions • Electrophilic addition • Nucleophilic addition – Substitution reactions • Electrophilic substitution • Nucleophilic substitution – Elimination reactions – Oxidation-reduction reactions – Isomerization reactions Copyright Mc. Graw-Hill 2009 69
Key Points • Polymers – Addition – Condensation – Biological • Proteins • Carbohydrates • Nucleic Acids Copyright Mc. Graw-Hill 2009 70
- Slides: 70