Basic Nuclear Physics 3 Modes of Radioactive Decay

Basic Nuclear Physics - 3 Modes of Radioactive Decay and Types of Radiation IAEA International Atomic Energy Agency Day 1 – Lecture 3

Objective Ø To understands modes of radioactive disintegration and types of radiation Ø To learn about basic atomic structure; alpha, beta, and gamma decay; positron emission; differences between gamma rays and x-rays; orbital electron capture; and internal conversion IAEA 2

Content Ø Basic atomic structure and isotopes Ø Alpha, beta, and gamma decay Ø Decay spectra Ø Differences between gamma rays and x-rays Ø Positron emission Ø Orbital electron capture Ø Internal conversion IAEA 3
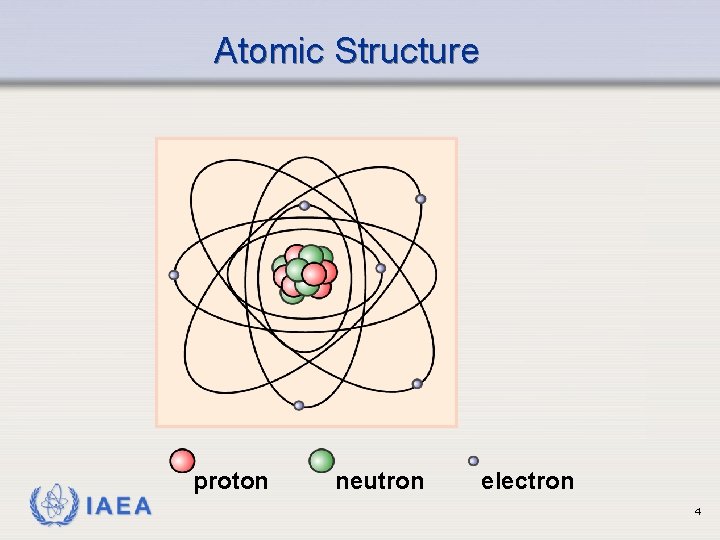
Atomic Structure IAEA proton neutron electron 4
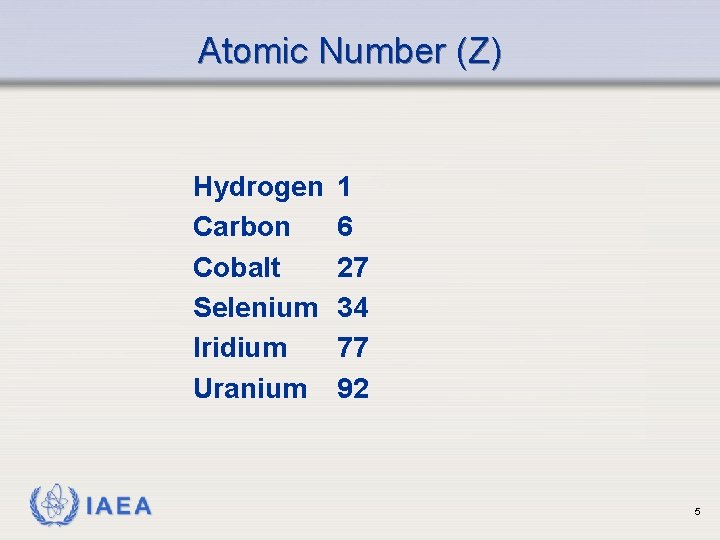
Atomic Number (Z) Hydrogen Carbon Cobalt Selenium Iridium Uranium IAEA 1 6 27 34 77 92 5
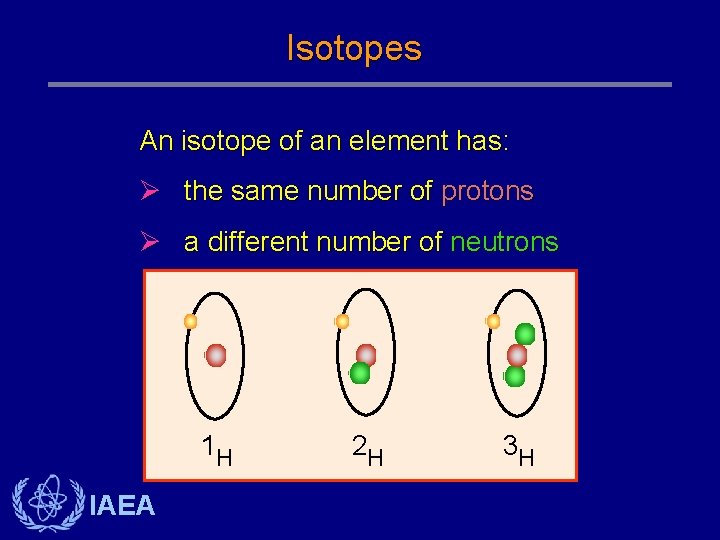
Isotopes An isotope of an element has: Ø the same number of protons Ø a different number of neutrons 1 H IAEA 2 H 3 H
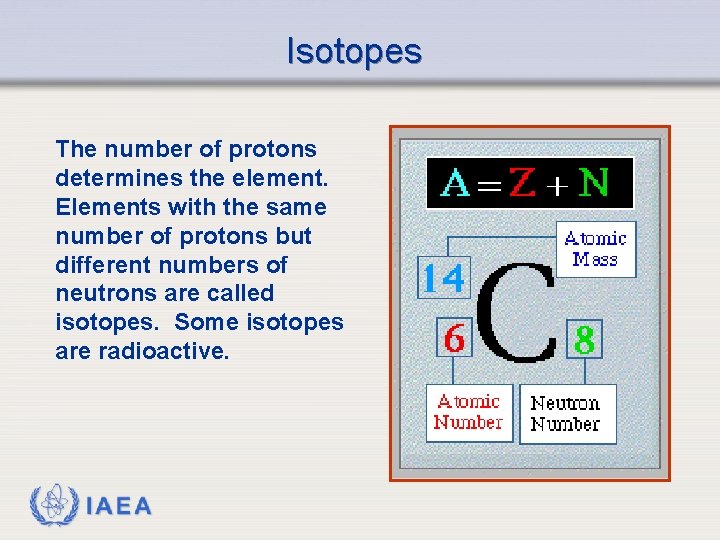
Isotopes The number of protons determines the element. Elements with the same number of protons but different numbers of neutrons are called isotopes. Some isotopes are radioactive. IAEA

Radioactive Decay Ø Spontaneous changes in the nucleus of an unstable atom Ø Results in formation of new elements Ø Accompanied by a release of energy, either particulate or electromagnetic or both Ø Nuclear instability is related to whether the neutron to proton ratio is too high or too low IAEA 8
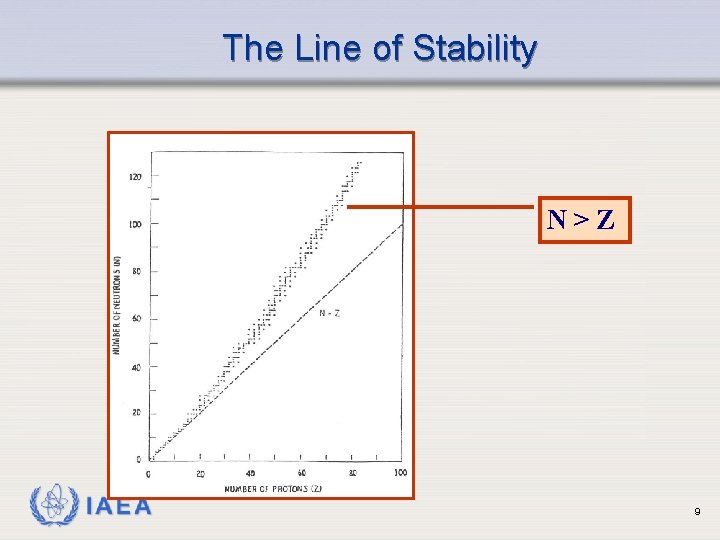
The Line of Stability N>Z IAEA 9
Alpha Emission Ø Emission of a highly energetic helium nucleus from the nucleus of a radioactive atom Ø Occurs when neutron to proton ratio is too low Ø Results in a decay product whose atomic number is 2 less than the parent and whose atomic mass is 4 less than the parent Ø Alpha particles are monoenergetic IAEA 10
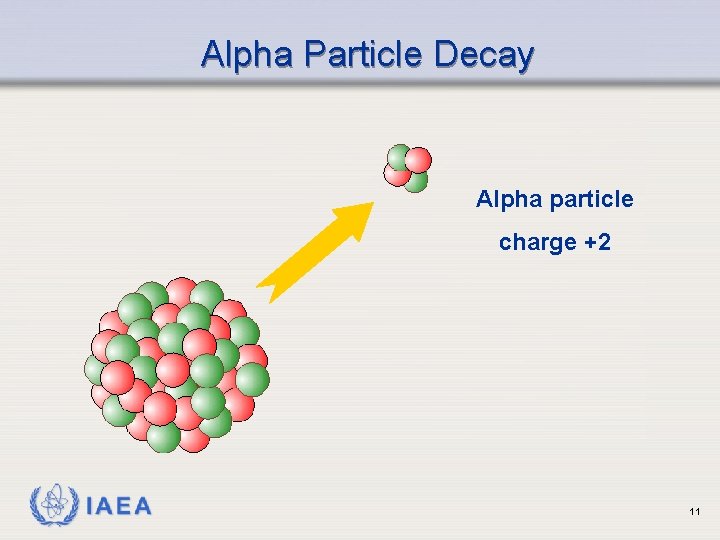
Alpha Particle Decay Alpha particle charge +2 IAEA 11
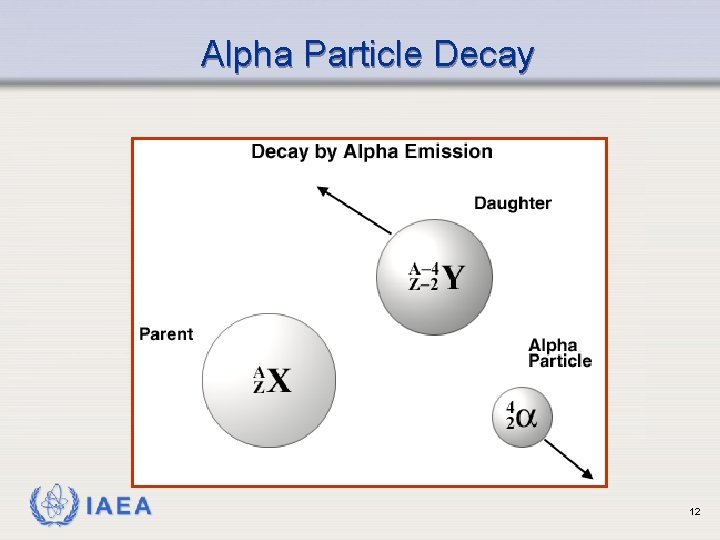
Alpha Particle Decay IAEA 12

Alpha Decay Example 226 Ra decays by alpha emission When 226 Ra decays, the atomic mass decreases by 4 and the atomic number decreases by 2 The atomic number defines the element, so the element changes from radium to radon 226 Ra 88 IAEA 222 Rn 86 + 42 He 13
Beta Emission Ø Emission of an electron from the nucleus of a radioactive atom ( n p+ + e-1 ) Ø Occurs when neutron to proton ratio is too high (i. e. , a surplus of neutrons) Ø Beta particles are emitted with a whole spectrum of energies (unlike alpha particles) IAEA 14
Beta Particle Decay Beta particle charge -1 IAEA 15
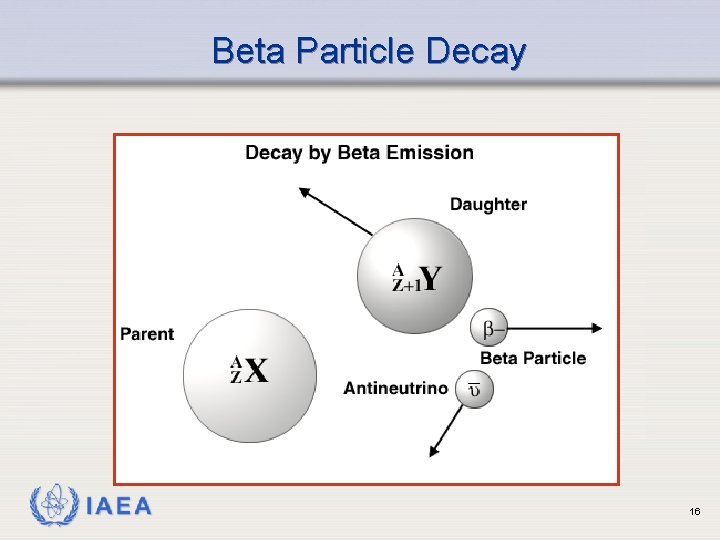
Beta Particle Decay IAEA 16
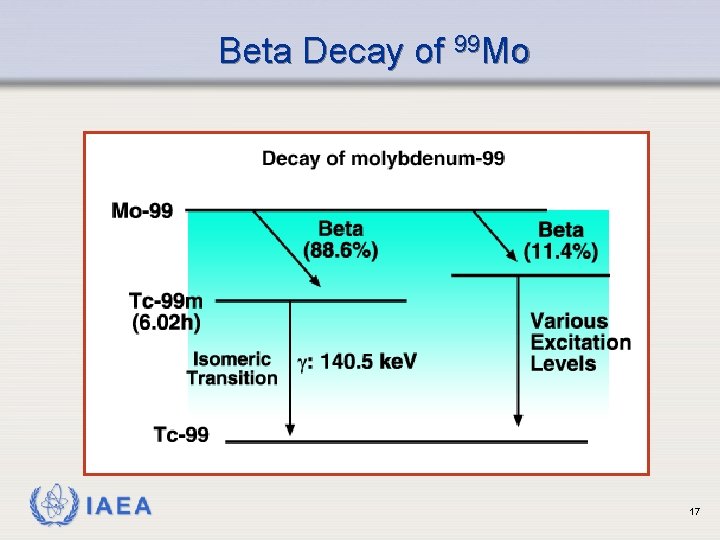
Beta Decay of 99 Mo IAEA 17
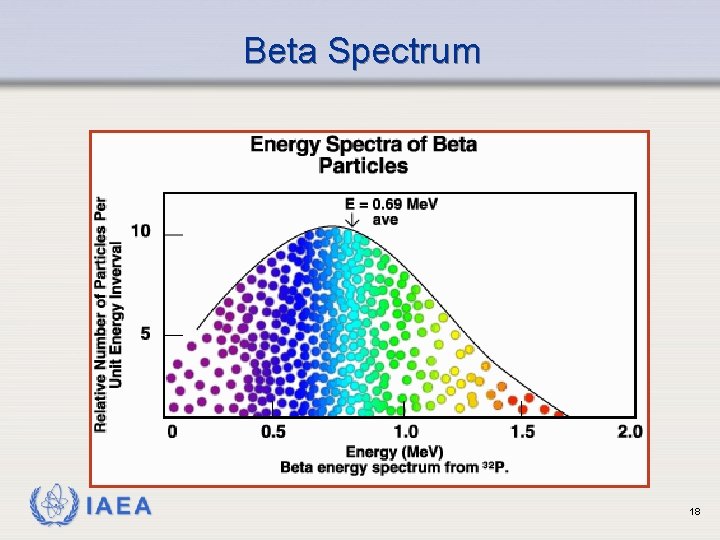
Beta Spectrum IAEA 18
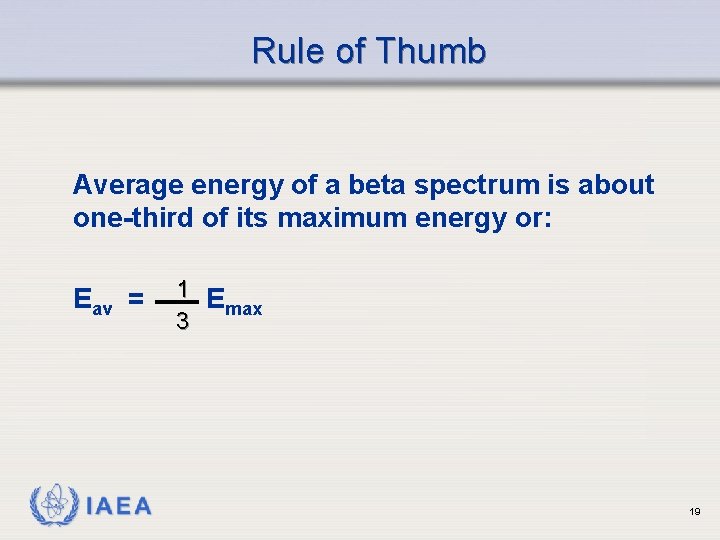
Rule of Thumb Average energy of a beta spectrum is about one-third of its maximum energy or: Eav = IAEA 1 E max 3 19
Positron (Beta+) Emission Ø Occurs when neutron to proton ratio is too low ( p+ n + e+ ) Ø Emits a positron (beta particle whose charge is positive) Ø Results in emission of 2 gamma rays (more on this later) IAEA 20
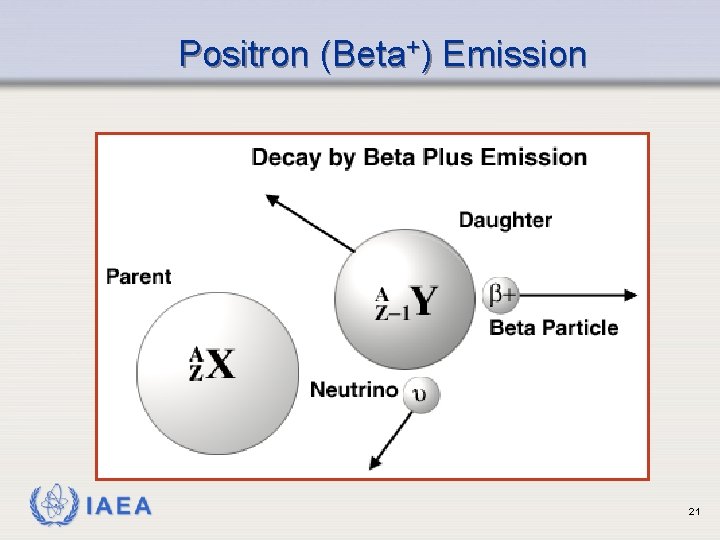
Positron (Beta+) Emission IAEA 21
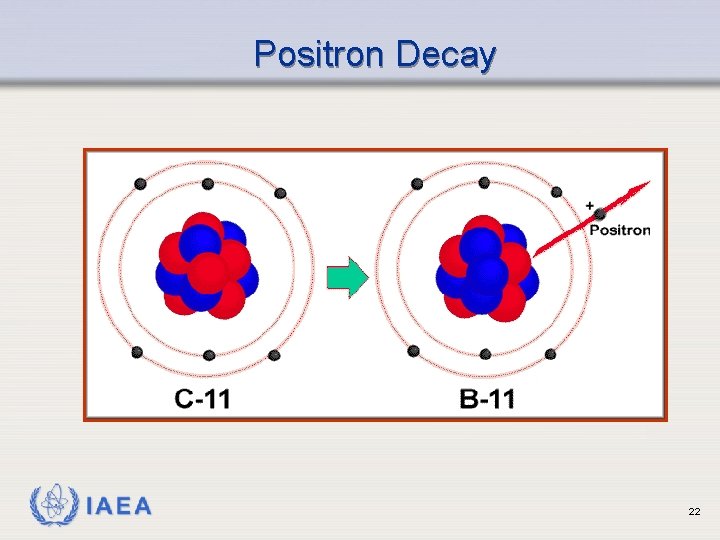
Positron Decay IAEA 22
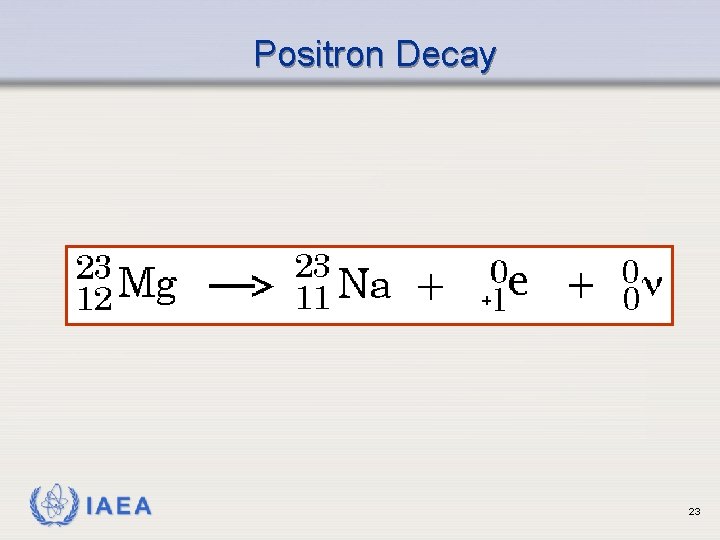
Positron Decay IAEA 23
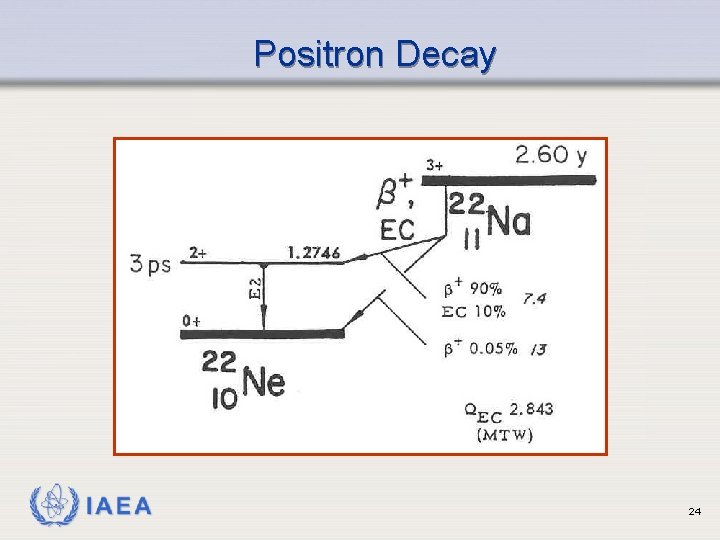
Positron Decay IAEA 24
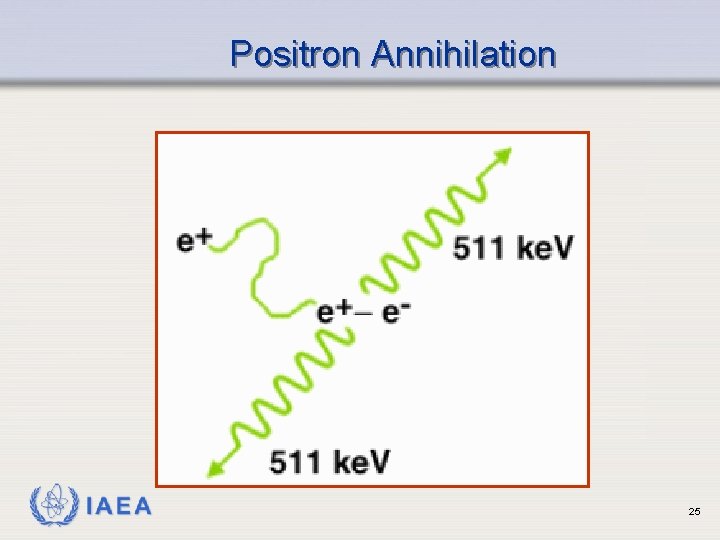
Positron Annihilation IAEA 25
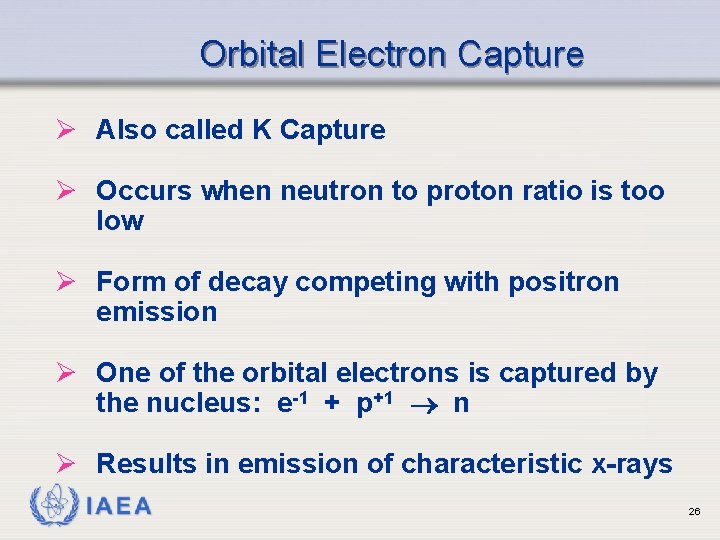
Orbital Electron Capture Ø Also called K Capture Ø Occurs when neutron to proton ratio is too low Ø Form of decay competing with positron emission Ø One of the orbital electrons is captured by the nucleus: e-1 + p+1 n Ø Results in emission of characteristic x-rays IAEA 26
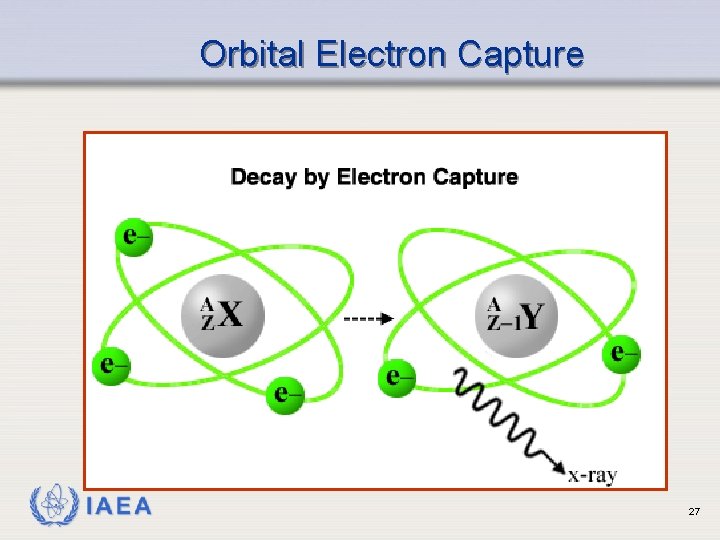
Orbital Electron Capture IAEA 27
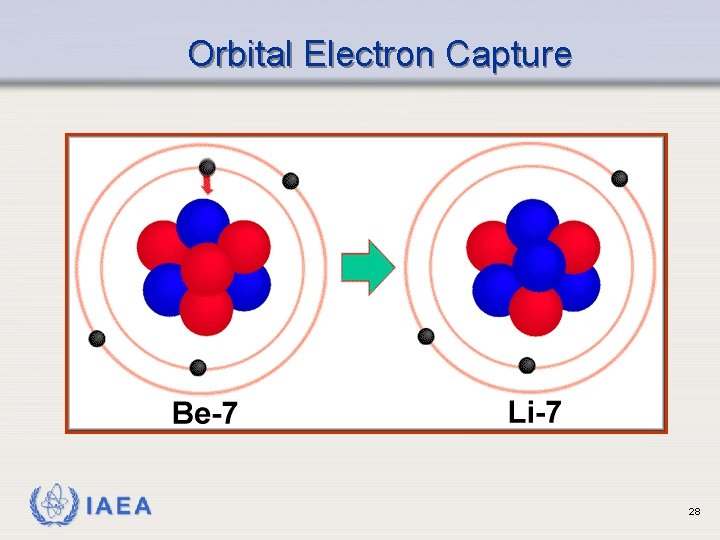
Orbital Electron Capture IAEA 28
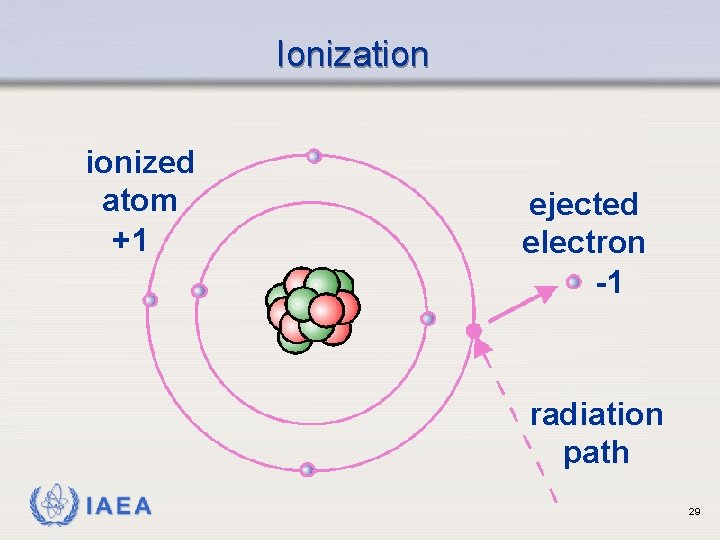
Ionization ionized atom +1 ejected electron -1 radiation path IAEA 29
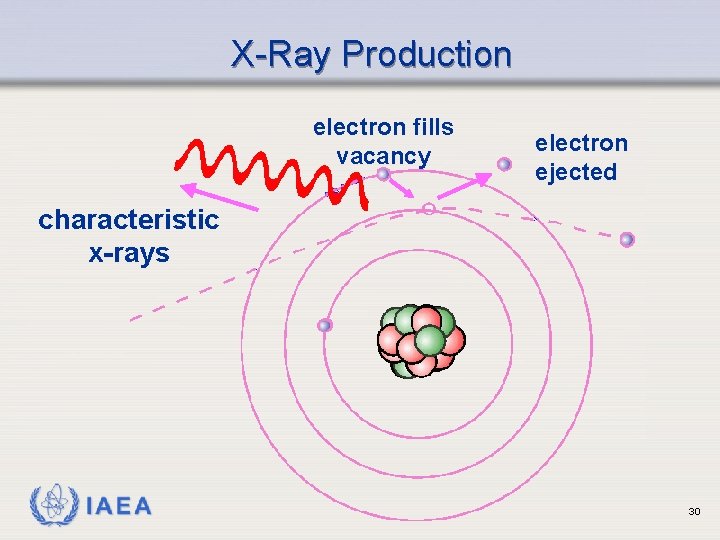
X-Ray Production electron fills vacancy electron ejected characteristic x-rays IAEA 30
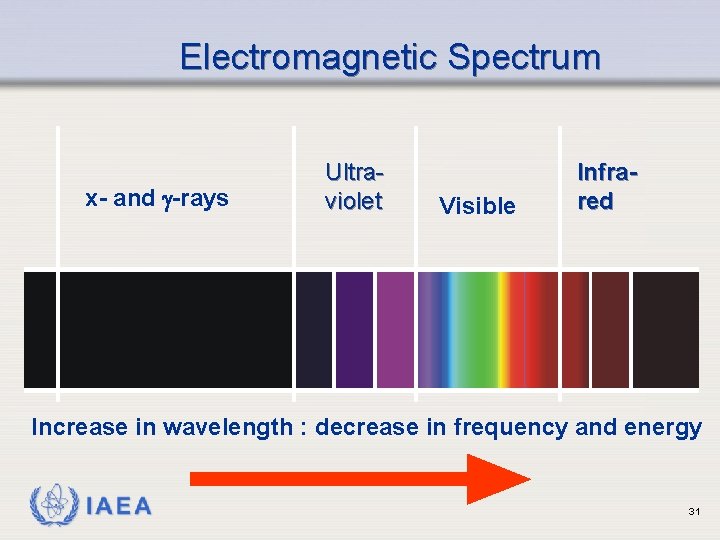
Electromagnetic Spectrum x- and -rays Ultraviolet Visible Infrared Increase in wavelength : decrease in frequency and energy IAEA 31
Gamma Ray Emission Ø Monoenergetic radiations emitted from nucleus of an excited atom following radioactive decay Ø Rid nucleus of excess energy Ø Have characteristic energies which can be used to identify the radionuclide Ø Excited forms of radionuclides often referred to as “metastable”, e. g. , 99 m. Tc. Also called “isomers” IAEA 32
Gamma Ray Emission Gamma Radiation IAEA 33
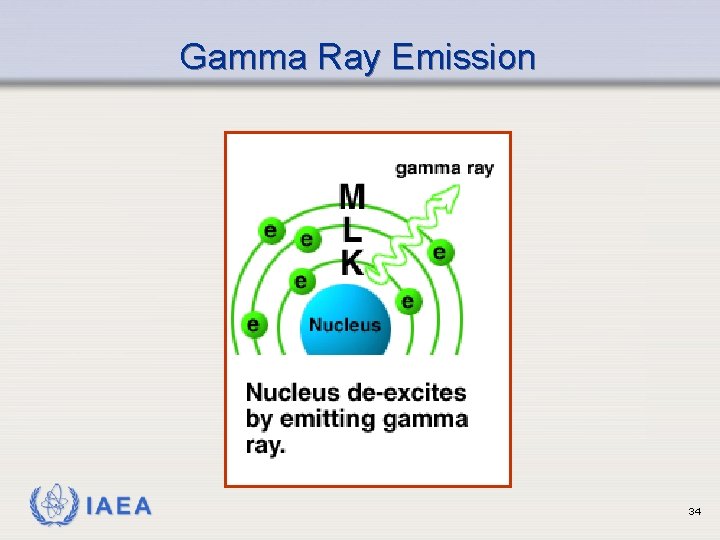
Gamma Ray Emission IAEA 34
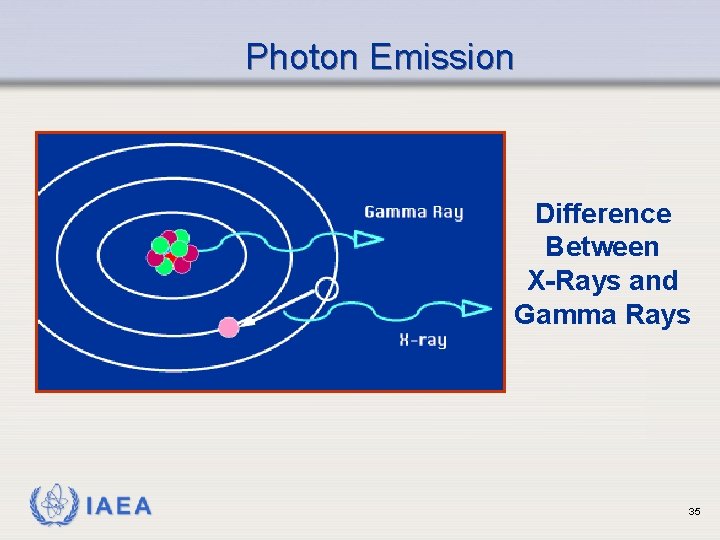
Photon Emission Difference Between X-Rays and Gamma Rays IAEA 35

Internal Conversion Ø Alternative process by which excited nucleus of a gamma emitting isotope rids itself of excitation energy Ø The nucleus emits a gamma ray which interacts with an orbital electron, ejecting the electron from the atom Ø Characteristic x-rays are emitted as outer orbital electrons fill the vacancies left by the conversion electrons IAEA 36

Internal Conversion Ø These characteristic x-rays can themselves be absorbed by orbital electrons, ejecting them. Ø These ejected electrons are called Auger electrons and have very little kinetic energy IAEA 37
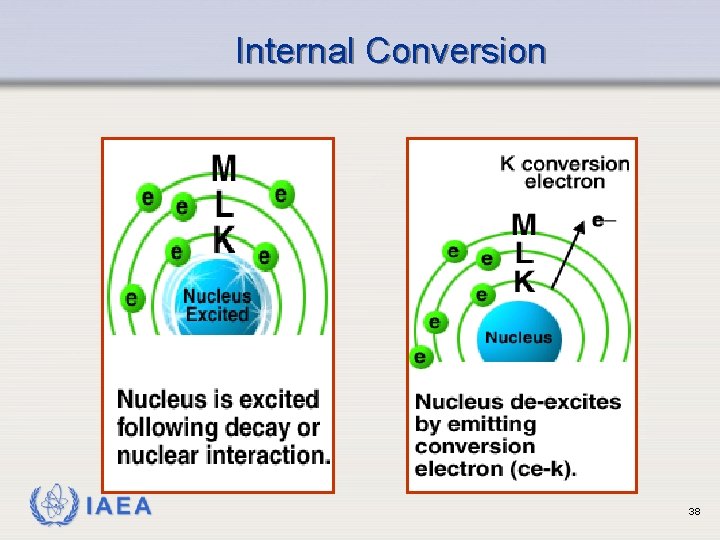
Internal Conversion IAEA 38
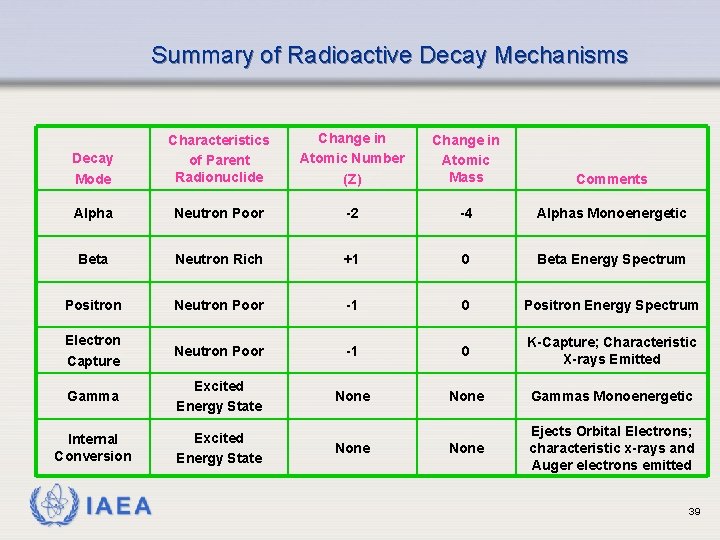
Summary of Radioactive Decay Mechanisms Decay Mode Characteristics of Parent Radionuclide Change in Atomic Number (Z) Change in Atomic Mass Comments Alpha Neutron Poor -2 -4 Alphas Monoenergetic Beta Neutron Rich +1 0 Beta Energy Spectrum Positron Neutron Poor -1 0 Positron Energy Spectrum Electron Capture Neutron Poor -1 0 K-Capture; Characteristic X-rays Emitted Gamma Excited Energy State None Gammas Monoenergetic Internal Conversion Excited Energy State None Ejects Orbital Electrons; characteristic x-rays and Auger electrons emitted IAEA None 39

Summary Ø Basic atomic structure was described Ø Isotopes were defined Ø Modes of radioactive disintegration were discussed (including alpha, beta, gamma, positron emission, orbital electron capture, and internal conversion) Ø Ionization was defined Ø X-ray production and the differences between gamma rays and x-rays were described IAEA 40

Where to Get More Information Ø Cember, H. , Johnson, T. E, Introduction to Health Physics, 4 th Edition, Mc. Graw-Hill, New York (2009) Ø International Atomic Energy Agency, Postgraduate Educational Course in Radiation Protection and the Safety of Radiation Sources (PGEC), Training Course Series 18, IAEA, Vienna (2002) IAEA 41
- Slides: 41