Basic Laws and Important People Intro to Chemistry

Basic Laws and Important People Intro to Chemistry

Fundamental Chemical Laws • 1. Law of Conservation of Mass • States that matter cannot be created or destroyed • Its just rearranged through chemical processes • Total mass of reactants must be equal to the total mass of the products


• 2. Law of Definite Proportion • A given compound always contains the same proportions of elements by mass • Compounds do not have a changing form • Compounds have a fixed ratio of components

Law of Multiple Proportions • When two elements form a series of compounds (Homologous series in organic) the ratio of masses of the elements can always be reduced to the smallest whole number • Also it is important to note that sometimes two elements can produce a series that is not able to be reduced • CO, CO 2, CO 3, etc…

Early Experiments to Characterize the Atom IMPORTANT PEOPLE AND WHAT THEY DID

J. J Thomson and Electrons • Determined the charge to mass ratio of the electron • - 1. 76 x 108 C/g • Reasoned that all atoms must contain at least 1 electron • Reasoned that atoms must have a positive charge as well although he didn’t “find” the particle • Used the cathode ray tube experiment to determine this information


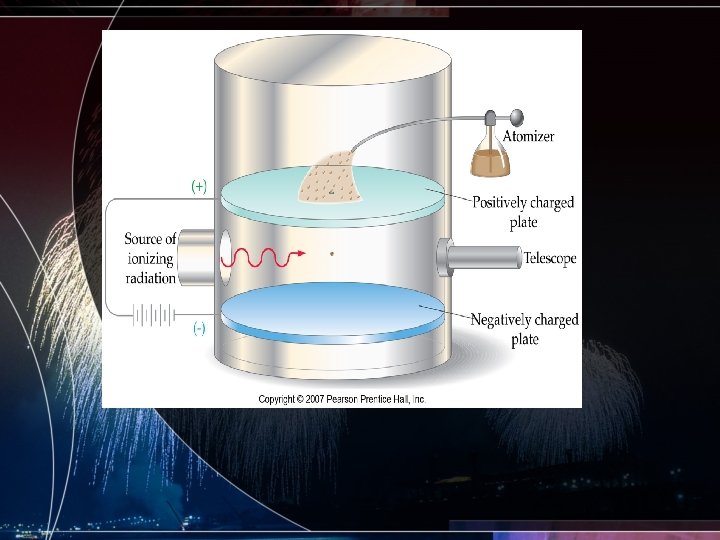
Robert Milikan and the oil drop • Used this experiment to determine the magnitude of the charge of an electron • Along with Thomson he determines the mass of an electron to be 9. 11 x 10 -31 kg

Radioactivity • Gamma Rays • Beta Particles • Alpha Particles

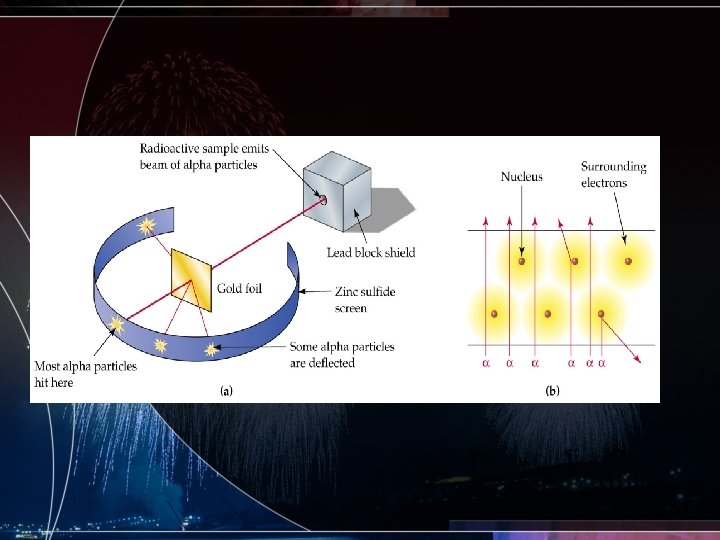
Rutherford and the Gold Foil Experiment • Uses this experiment to determine that all atoms are composed of mostly empty space • All atoms also have a positive dense core that he calls a nucleus • Makes an attempt to develop an atomic model but was incorrect with his placement of particles • Considered the ground breaking experiment in the fields of chemistry and physics with regards to atoms



2. 5 Modern View of The Atom • 1. Nucleus---center of mass • Contains protons and neutrons • Positive charge that is indicated by the atomic number of an element • Small size, large density • 2. Electrons • Source of reactivity • Negative charge • 1/2000 th the mass of a proton

Parts of an atom Continued • • 3. Atomic Number– same as number of protons Identifying feature of all elements Number cannot be changed for an individual atom 4. Mass Number Protons + Neutrons Can be different for isotopes of the same element Number on periodic table is a weighted average of all known isotopes for the element

Parts…. • 5. Isotopes----same element with a different number of neutrons • Gives this version a different mass
YOUR TOPIC GOES HERE • Your Subtopics Go Here

YOUR TOPIC GOES HERE • Your Subtopics Go Here

TRANSITIONAL PAGE

Template Provided By www. animationfactory. com 500, 000 Downloadable Power. Point Templates, Animated Clip Art, Backgrounds and Videos
- Slides: 24