Basic Glass theory Traditional Glass former flux modifier
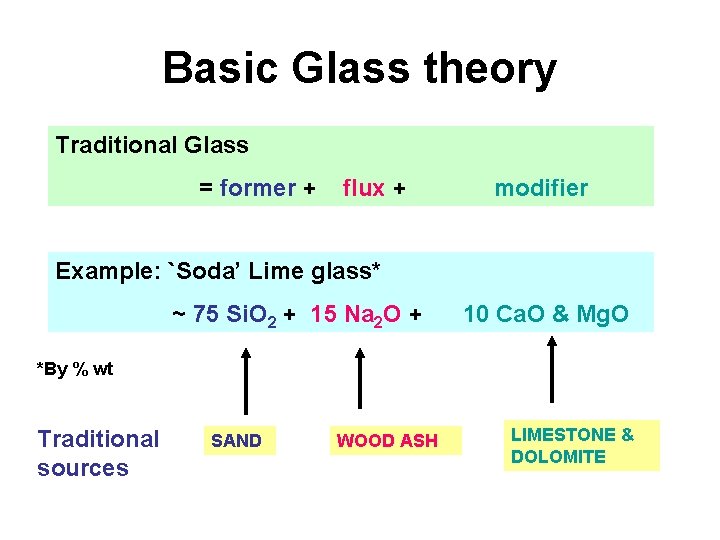
Basic Glass theory Traditional Glass = former + flux + modifier Example: `Soda’ Lime glass* ~ 75 Si. O 2 + 15 Na 2 O + 10 Ca. O & Mg. O *By % wt Traditional sources SAND WOOD ASH LIMESTONE & DOLOMITE
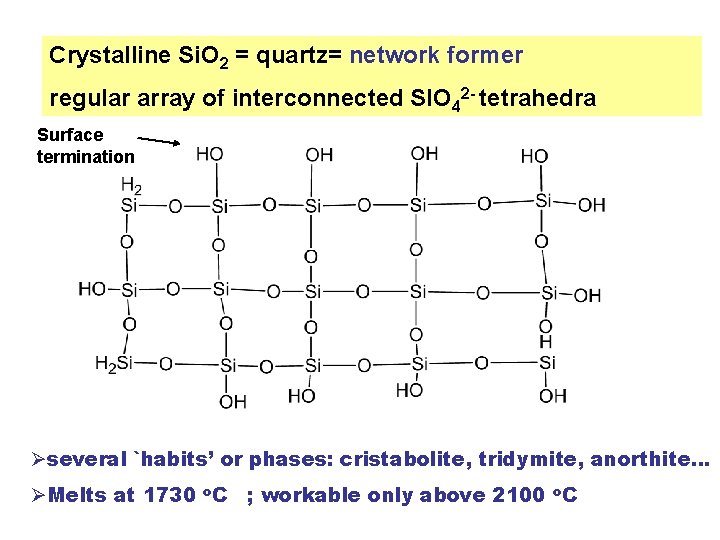
Crystalline Si. O 2 = quartz= network former regular array of interconnected SIO 42 - tetrahedra Surface termination Øseveral `habits’ or phases: cristabolite, tridymite, anorthite… ØMelts at 1730 o. C ; workable only above 2100 o. C
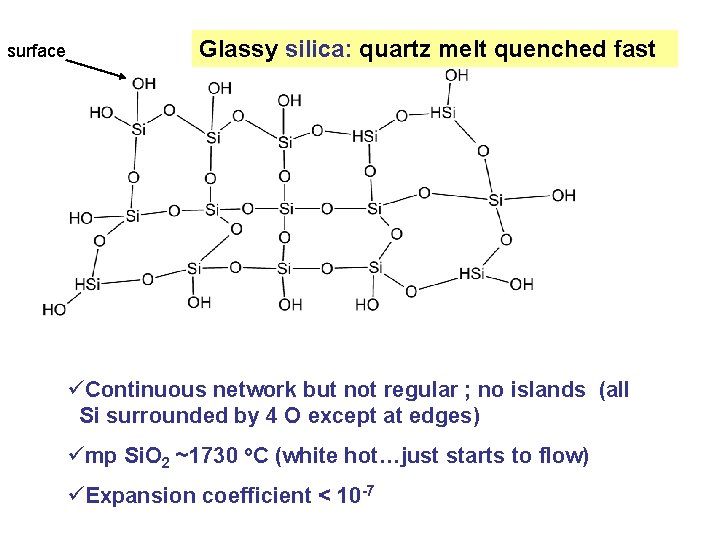
surface Glassy silica: quartz melt quenched fast üContinuous network but not regular ; no islands (all Si surrounded by 4 O except at edges) ümp Si. O 2 ~1730 o. C (white hot…just starts to flow) üExpansion coefficient < 10 -7
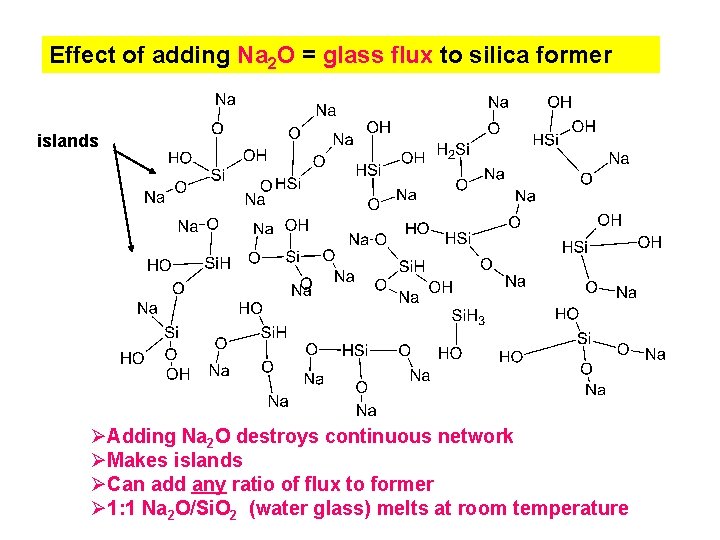
Effect of adding Na 2 O = glass flux to silica former islands ØAdding Na 2 O destroys continuous network ØMakes islands ØCan add any ratio of flux to former Ø 1: 1 Na 2 O/Si. O 2 (water glass) melts at room temperature
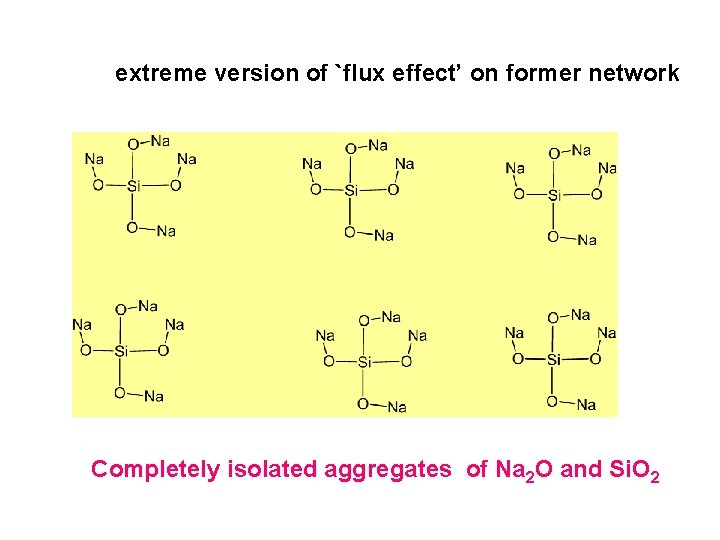
extreme version of `flux effect’ on former network Completely isolated aggregates of Na 2 O and Si. O 2

EFFECT OF MODIFIER Flux + former No modifier MP < 100 O C Add Ca. O Addition of modifier reconnects some islands (heals Na 2 O disruption) MP ~1200 OC

Compositional effect summarized MP o. C expansion Pure former (Si. O 2) 1730 Very low Almost nil 50: 50 Na 2 O/Si. O 2 100 Very high Dissolves in water moderate conc. Na. OH (flux + former) ~3150 o F 15: 10: 75 Na 2 O/Ca. O/ Si. O 2 (flux + modifier + former) 1200 reactivity

Where glass making diverges from standard chemistry Can vary ratios of flux, former and modifier continuously and non-stoichiometrically !! => infinite number of glasses possible for just three components `standard’ chemistry: only whole numbered ratios of components ok 1: 1: 1 Na 2 O/Ca. O/Si. O 2 1: 1: 4 Na 2 O/Ca. O/Si. O 2 Glass chemistry; any non-whole number ratio of components can perhaps make a glass 1. 65: 0. 30: 3. 6 0. 8: 0. 25: 6. 0 Na 2 O/Ca. O/Si. O 2

MANY WAYS TO CHARACTERIZE GLASS …THREE MAJOR WORKHORSES ARE: 1) Differential scanning Calorimetry (DSC): • measures glass transition and glass crystallization temperatures (Tg and Tc) 2) X-ray diffractometry (XRD): • Measures extent of crystallinity and devitrification products in glass; allows study of crystal structures in glass 3) Scanning Electron Microscopy • Surface structure, local composition (SEM):
- Slides: 9