Basic Concepts of Electrochemical Cells Anode Cathode 1
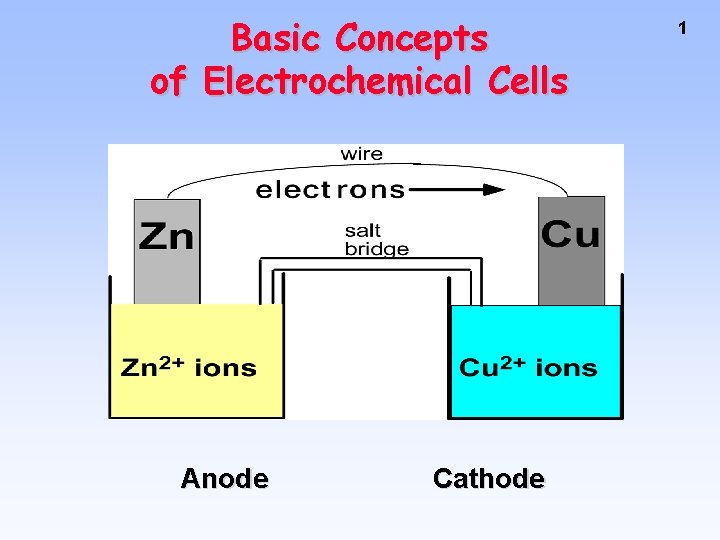
Basic Concepts of Electrochemical Cells Anode Cathode 1
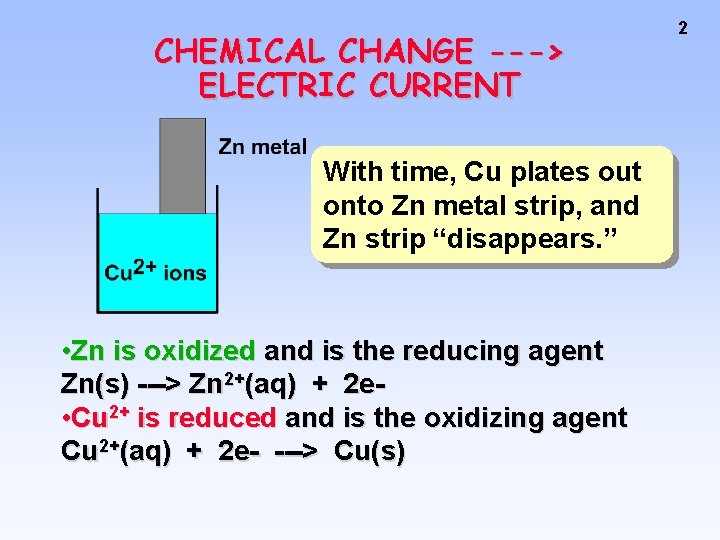
CHEMICAL CHANGE ---> ELECTRIC CURRENT With time, Cu plates out onto Zn metal strip, and Zn strip “disappears. ” • Zn is oxidized and is the reducing agent Zn(s) ---> Zn 2+(aq) + 2 e • Cu 2+ is reduced and is the oxidizing agent Cu 2+(aq) + 2 e- ---> Cu(s) 2
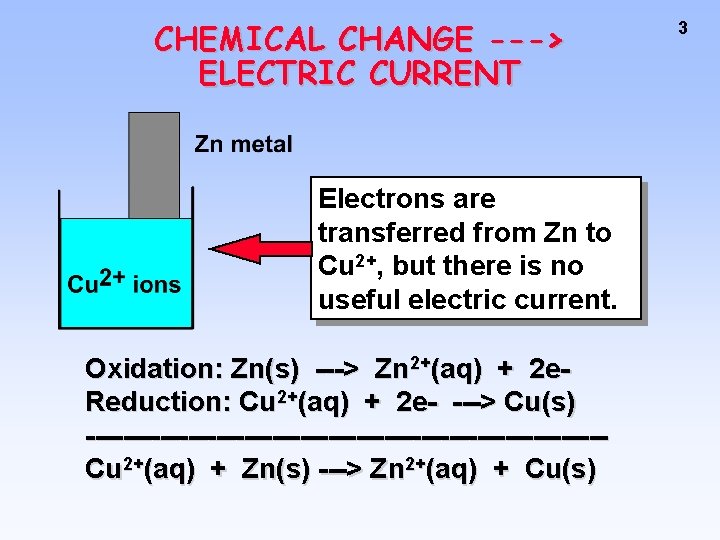
CHEMICAL CHANGE ---> ELECTRIC CURRENT Electrons are transferred from Zn to Cu 2+, but there is no useful electric current. Oxidation: Zn(s) ---> Zn 2+(aq) + 2 e. Reduction: Cu 2+(aq) + 2 e- ---> Cu(s) ----------------------------Cu 2+(aq) + Zn(s) ---> Zn 2+(aq) + Cu(s) 3
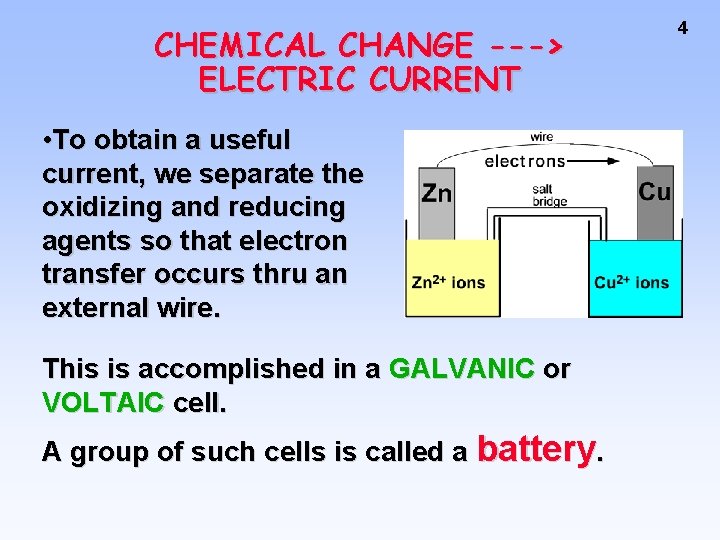
CHEMICAL CHANGE ---> ELECTRIC CURRENT • To obtain a useful current, we separate the oxidizing and reducing agents so that electron transfer occurs thru an external wire. This is accomplished in a GALVANIC or VOLTAIC cell. A group of such cells is called a battery. 4
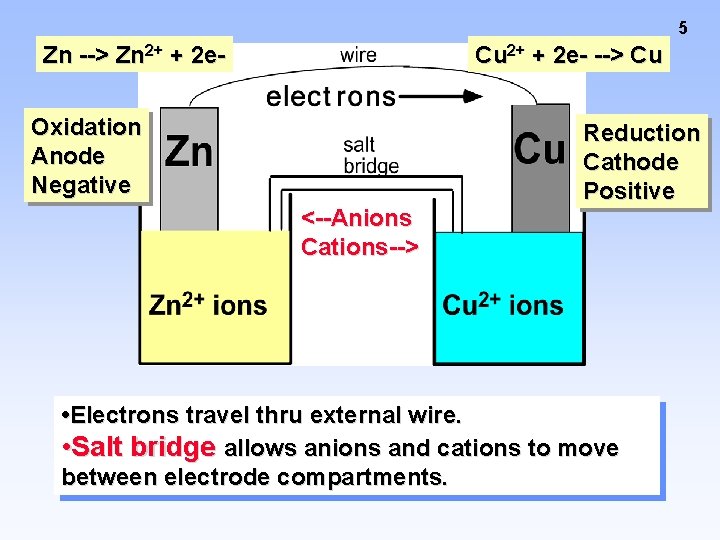
5 Zn --> Zn 2+ + 2 e- Cu 2+ + 2 e- --> Cu Oxidation Anode Negative <--Anions Cations--> Reduction Cathode Positive • Electrons travel thru external wire. • Salt bridge allows anions and cations to move between electrode compartments.
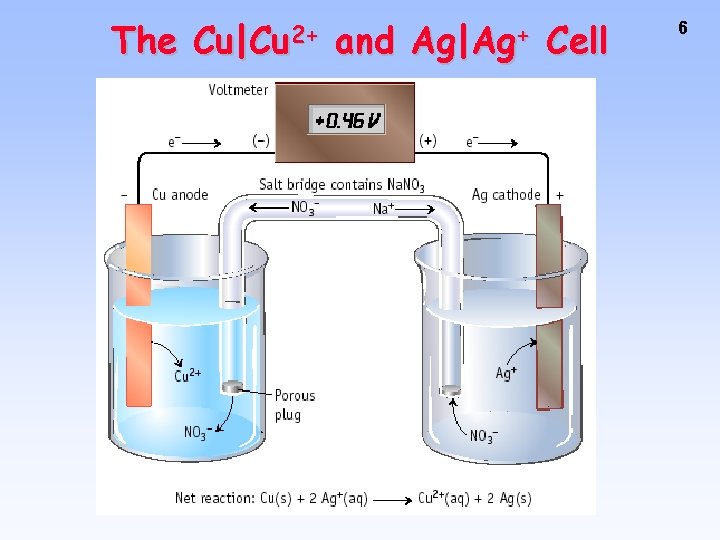
The Cu|Cu 2+ and Ag|Ag+ Cell 6
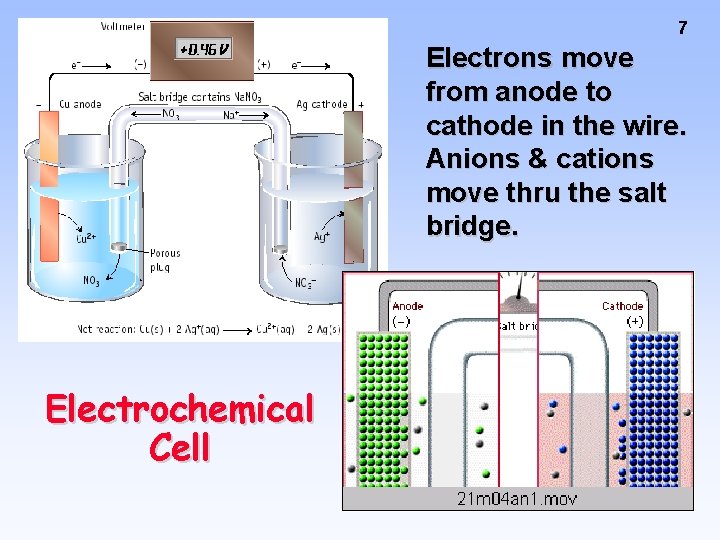
7 Electrons move from anode to cathode in the wire. Anions & cations move thru the salt bridge. Electrochemical Cell
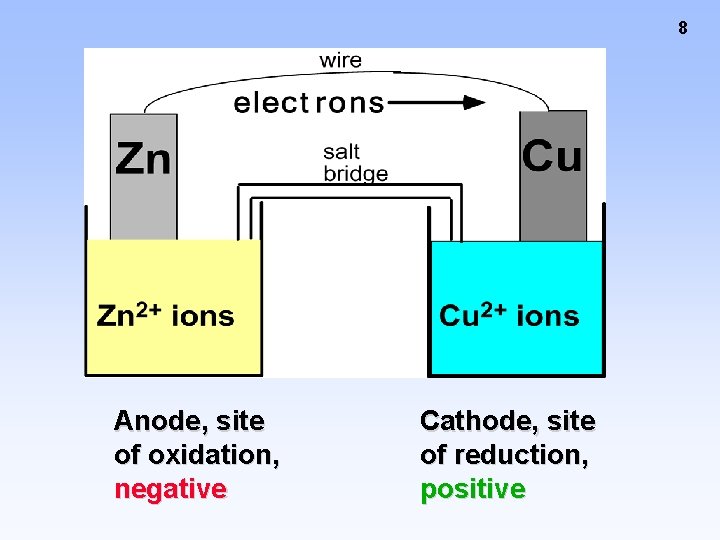
8 Anode, site of oxidation, negative Cathode, site of reduction, positive
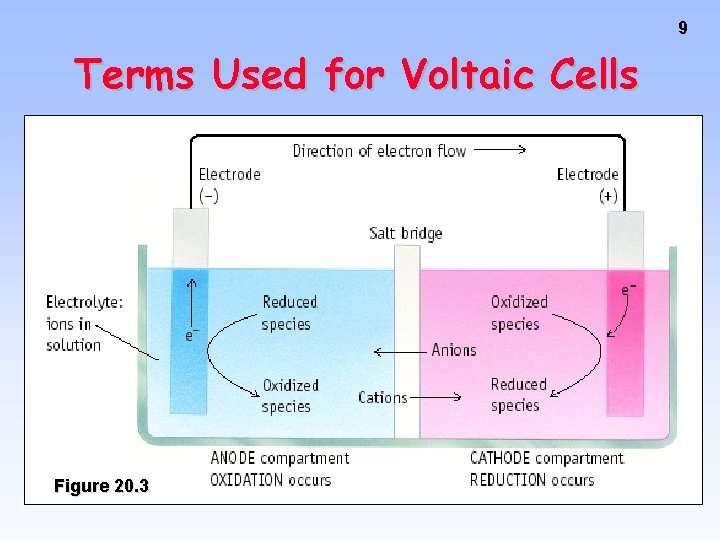
9 Terms Used for Voltaic Cells Figure 20. 3
- Slides: 9