Basic Concepts in Protein Structure Pharmacy Biomedical Preview
Basic Concepts in Protein Structure Pharmacy Biomedical Preview Program, Day 1 Summer 2015 Nicole Catterlin, P 3

AKA: The Slideshow That Went On Forever (Sorry kiddos!)

What Are We Made of? ● Four Basic “Macromolecule” Families Lipids: energy and structure § butter, wax, cholesterol o Carbohydrates: energy § starch, sugar o Nucleic Acids: blueprints § DNA and RNA o Proteins: the amazing jack of all trades § MAGIC!!!! o

Proteins: Nature’s Legos ● Structure o Muscle, collagen, hair ● Movement o Muscles (Actin, Myosin) ● Enzymes Molecular Machines Promote chemical reactions in Anabolism and Catabolism o Life o o
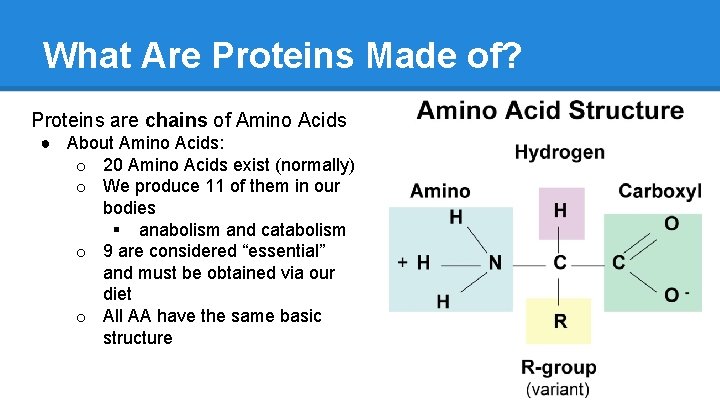
What Are Proteins Made of? Proteins are chains of Amino Acids ● About Amino Acids: o 20 Amino Acids exist (normally) o We produce 11 of them in our bodies § anabolism and catabolism o 9 are considered “essential” and must be obtained via our diet o All AA have the same basic structure
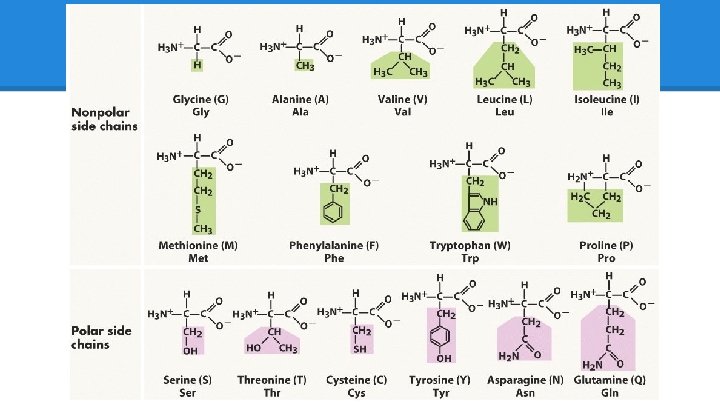
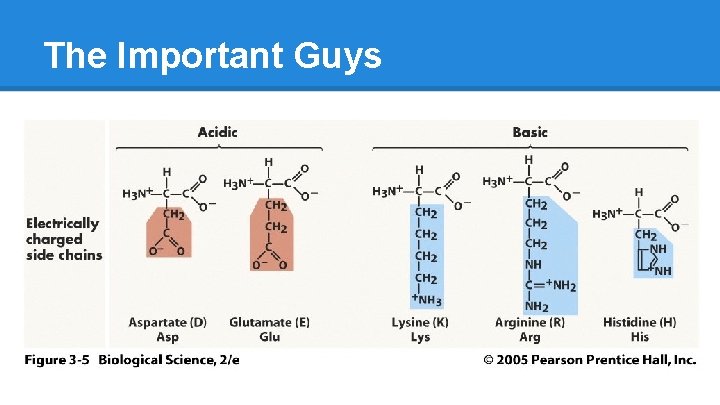
The Important Guys
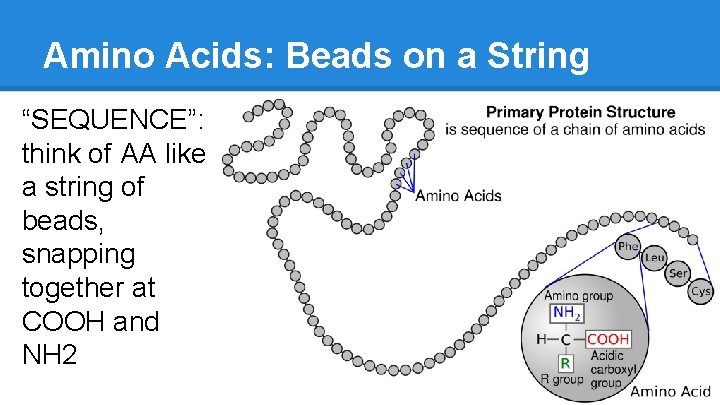
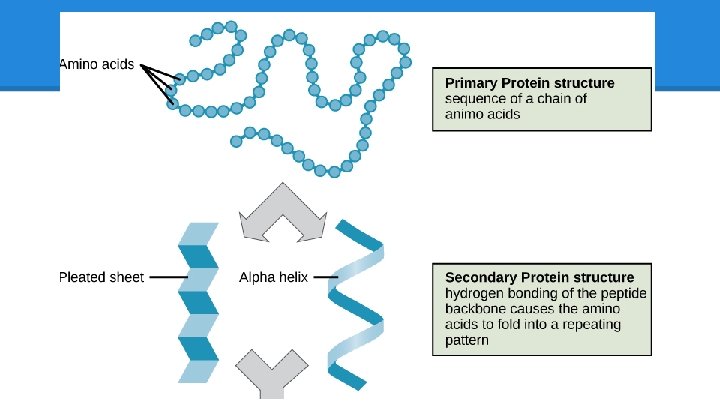
Amino Acids: Beads on a String “SEQUENCE”: think of AA like a string of beads, snapping together at COOH and NH 2
Protein Structure: Levels A string of AA is not a structure or a molecular machine. . . What else needs to happen to make this a reality?

Let’s make this instead. . .
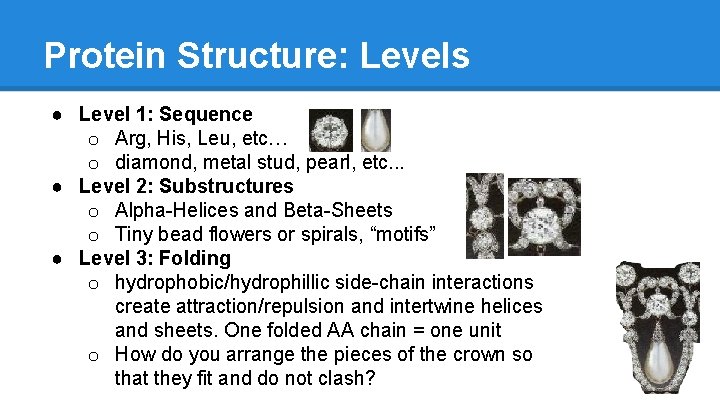
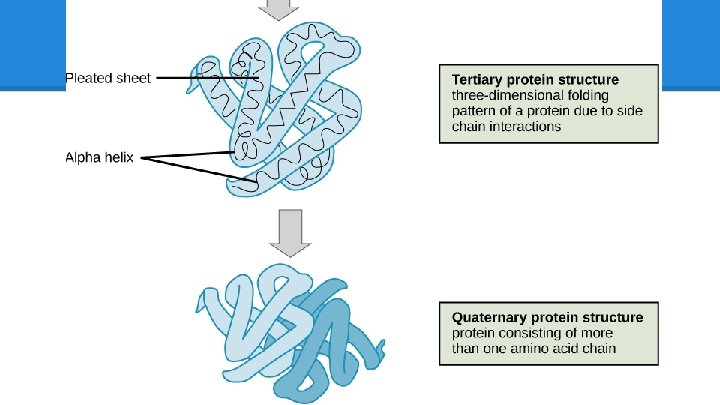
Protein Structure: Levels ● Level 1: Sequence o Arg, His, Leu, etc… o diamond, metal stud, pearl, etc. . . ● Level 2: Substructures o Alpha-Helices and Beta-Sheets o Tiny bead flowers or spirals, “motifs” ● Level 3: Folding o hydrophobic/hydrophillic side-chain interactions create attraction/repulsion and intertwine helices and sheets. One folded AA chain = one unit o How do you arrange the pieces of the crown so that they fit and do not clash?
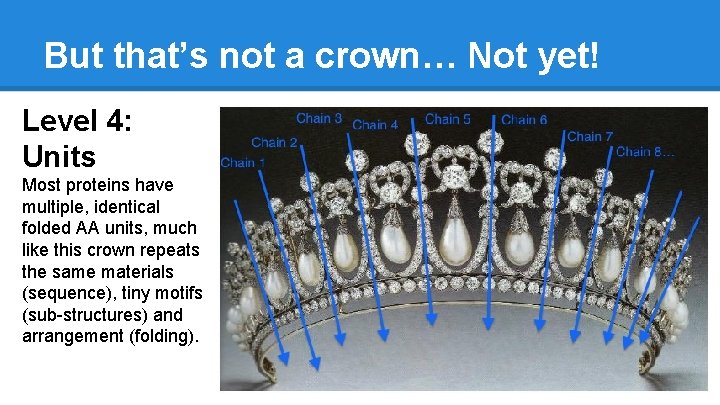
But that’s not a crown… Not yet! Level 4: Units Most proteins have multiple, identical folded AA units, much like this crown repeats the same materials (sequence), tiny motifs (sub-structures) and arrangement (folding).

How do Proteins Fold? ● Subject of A LOT of research o o Hard to predict Why? ● Hydrophobic and Hydrophillic interactions o We are an aqueous environment. What types of amino acids can interface easily with water? § Rope exercise
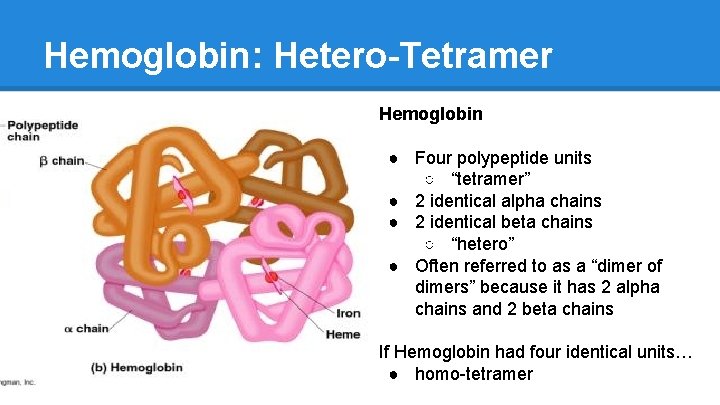
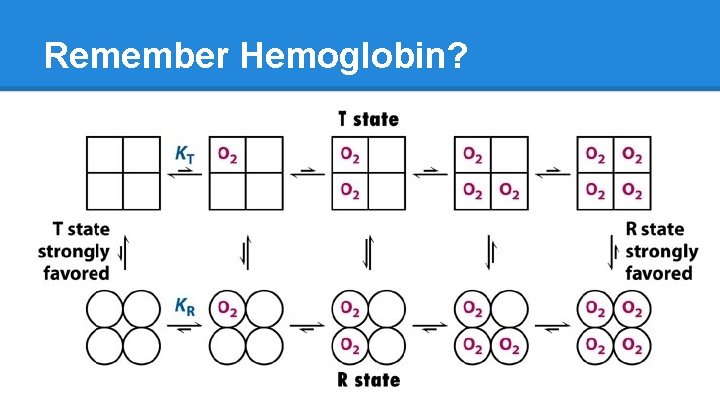
Hemoglobin: Hetero-Tetramer Hemoglobin ● Four polypeptide units ○ “tetramer” ● 2 identical alpha chains ● 2 identical beta chains ○ “hetero” ● Often referred to as a “dimer of dimers” because it has 2 alpha chains and 2 beta chains If Hemoglobin had four identical units… ● homo-tetramer
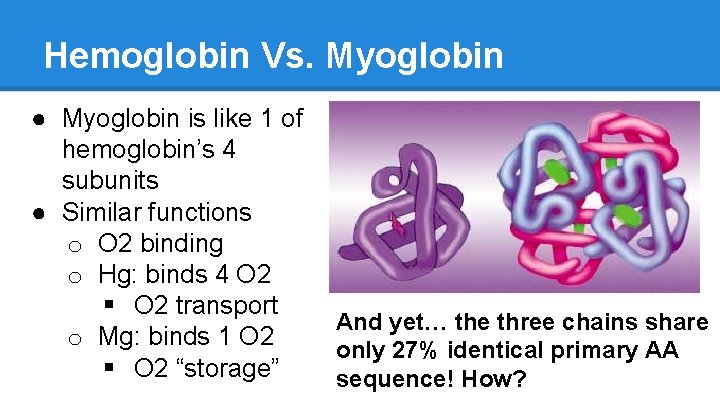
Hemoglobin Vs. Myoglobin ● Myoglobin is like 1 of hemoglobin’s 4 subunits ● Similar functions o O 2 binding o Hg: binds 4 O 2 § O 2 transport o Mg: binds 1 O 2 § O 2 “storage” And yet… the three chains share only 27% identical primary AA sequence! How?
The 27% Overlap: Conserved Sequences ● Only VERY few AA participate in the “function” of a given protein/enzyme ● Most AA are structural (shape) ● Conserved Sequences: Vital AA that determine folding and function o Ex: ARG 324, CYS 556, ASP 23 ● Why is this evolutionarily advantageous? o HINT: Protein sequences are coded by DNA. . .
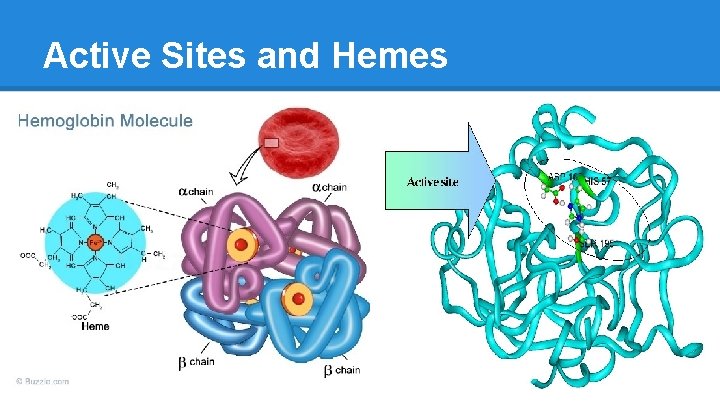
Active Sites and Hemes
Conserved Sequences ● In case of Hemoglobin, O 2 is bound by a Heme, or a “cofactor” with an Fe++ ion in the middle ● Amino Acids in the active site of an enzyme serve two roles o Orienting/Binding § molecule has to be properly angled to undergo molecular “surgery”, proper functional groups exposed o Catalysis § Transformation, by proper acid and base “encouragers” ● A few select Amino Acids are regularly found in Active Sites… Can you guess which and why? o hint: polar types, non-polar types, charged types (acid/base)
Proteins: Review Proteins are… ● made of amino acids ● Structural ● may have up to 4 Macromolecules levels of organization ○ skin, hair o Primary (sequence) ● Connective Tissue o Secondary (sheets and ○ collagen, RBC helixes) ● Enzymes o Tertiary (folding) o Quaternary (ind. chains) ○ amylase
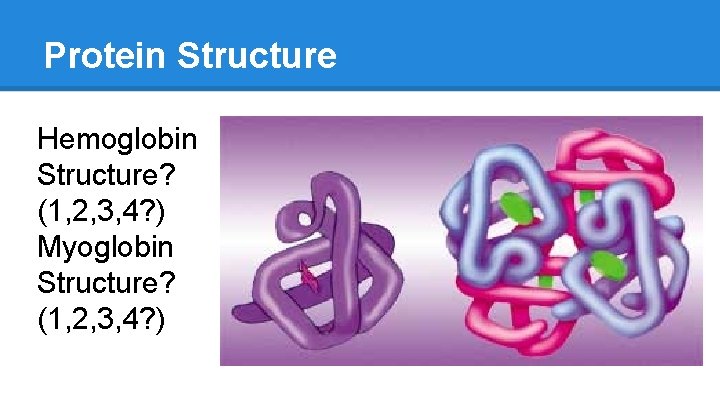
Protein Structure Hemoglobin Structure? (1, 2, 3, 4? ) Myoglobin Structure? (1, 2, 3, 4? )
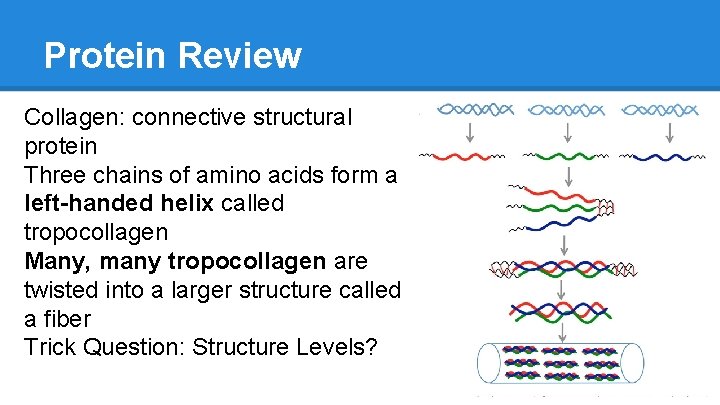
Protein Review Collagen: connective structural protein Three chains of amino acids form a left-handed helix called tropocollagen Many, many tropocollagen are twisted into a larger structure called a fiber Trick Question: Structure Levels?
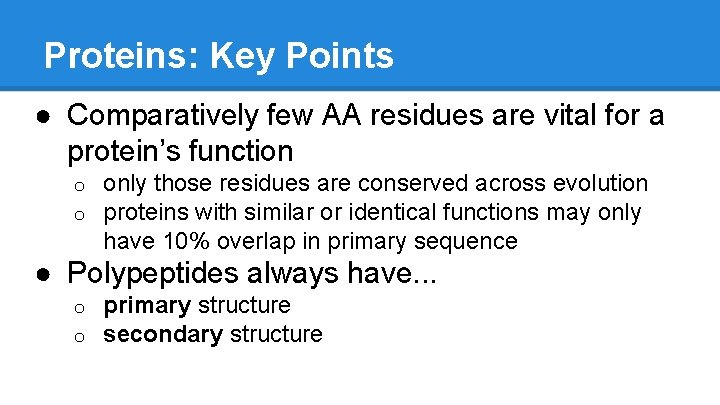
Proteins: Key Points ● Comparatively few AA residues are vital for a protein’s function o o only those residues are conserved across evolution proteins with similar or identical functions may only have 10% overlap in primary sequence ● Polypeptides always have. . . o o primary structure secondary structure
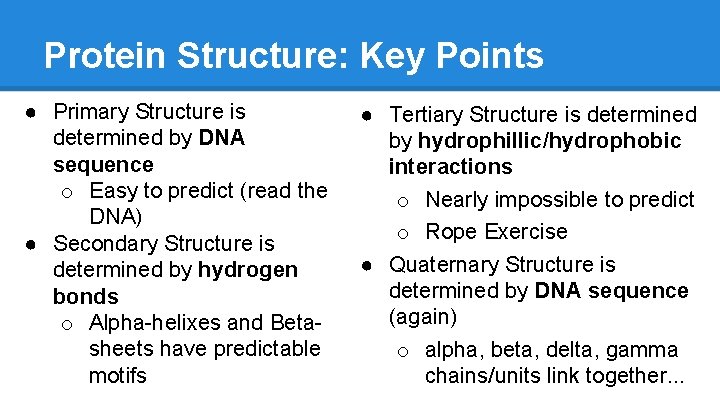
Protein Structure: Key Points ● Primary Structure is determined by DNA sequence o Easy to predict (read the DNA) ● Secondary Structure is determined by hydrogen bonds o Alpha-helixes and Betasheets have predictable motifs ● Tertiary Structure is determined by hydrophillic/hydrophobic interactions o Nearly impossible to predict o Rope Exercise ● Quaternary Structure is determined by DNA sequence (again) o alpha, beta, delta, gamma chains/units link together. . .
Enzymes: An Introduction Enzymes are proteins that act like molecular machines, also called Catalysts ● speed up chemically favored but slow processes o proximity, position, hydrophobic environment o can increase rate of rxn from 1 per hour to 1 x 10^9 per hour ● both anabolic and catabolic functions ● reversible and non-reversible

Enzymes: Examples ● Amylase o converts starch (polysaccharide) into simple sugars o ana/cata? ● Lipase o breaks down triglycerides into fatty acids o ana/cata? ● Aminotransferase o transfers an amino group from one molecule to another o ana/cata? ● DNA ligase o mends “nicks” in DNA strands after repair o ana/cata?
![Are You Catching a Name Trend? [-ase = enzyme] Remember it! Are You Catching a Name Trend? [-ase = enzyme] Remember it!](http://slidetodoc.com/presentation_image_h/c73d88d212f37394ff87ba288da3194f/image-28.jpg)
Are You Catching a Name Trend? [-ase = enzyme] Remember it!
![How do Enzymes work? [Substrate + Enzyme] ➡ S/E Complex ➡ Product ● binds How do Enzymes work? [Substrate + Enzyme] ➡ S/E Complex ➡ Product ● binds](http://slidetodoc.com/presentation_image_h/c73d88d212f37394ff87ba288da3194f/image-29.jpg)
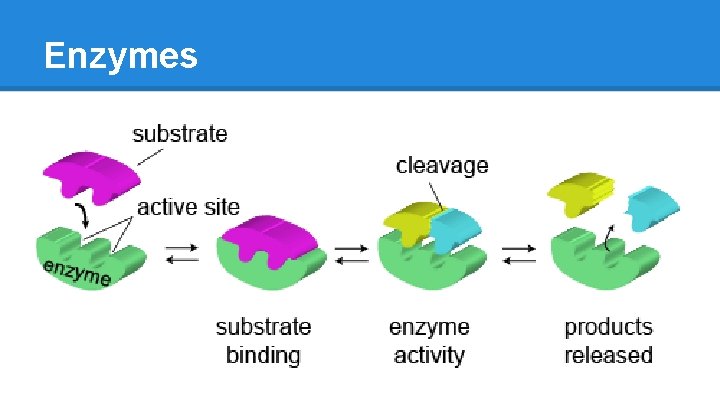
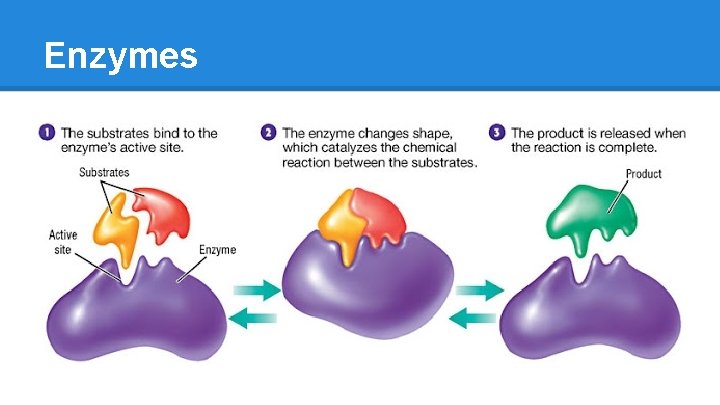
How do Enzymes work? [Substrate + Enzyme] ➡ S/E Complex ➡ Product ● binds SPECIFIC substrates o brings them into proximity o brings them into proper position o hydrophobic (water exclusionary) environment ● self-regenerating o any altered AA residues are restored by the end by acid-base interactions ● transcribed and translated from genes o DNA > RNA > translation via ribosomes o translation can be “up-regulated” or “down-regulated” according to body’s needs
Enzymes
Enzymes
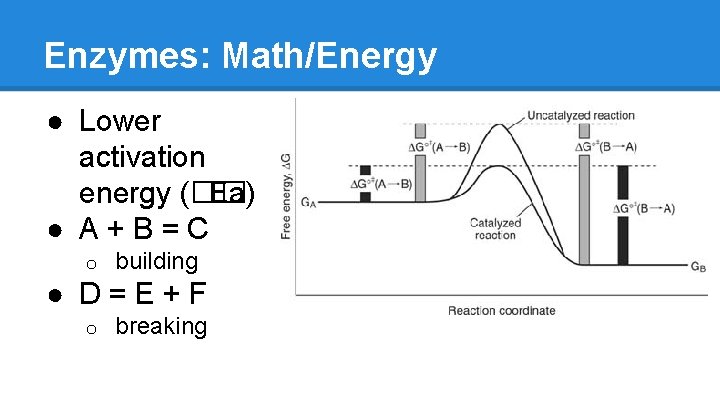
Enzymes: Math/Energy ● Lower activation energy (�� Ea) ● A+B=C o building ● D=E+F o breaking
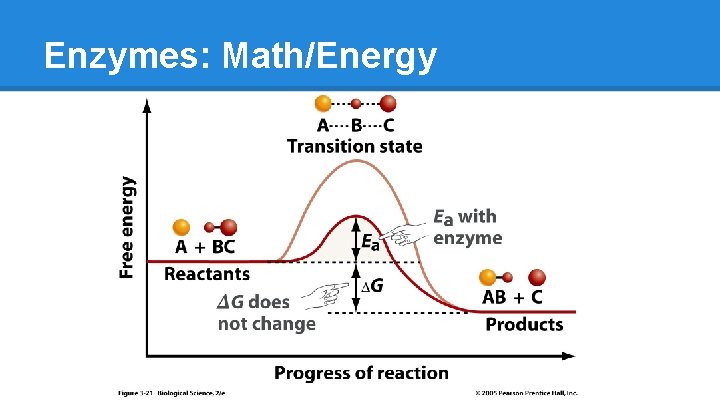
Enzymes: Math/Energy
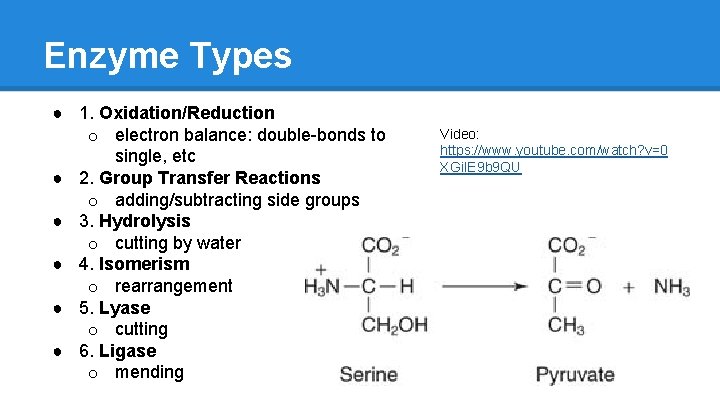
Enzyme Types ● 1. Oxidation/Reduction o electron balance: double-bonds to single, etc ● 2. Group Transfer Reactions o adding/subtracting side groups ● 3. Hydrolysis o cutting by water ● 4. Isomerism o rearrangement ● 5. Lyase o cutting ● 6. Ligase o mending Video: https: //www. youtube. com/watch? v=0 XGi. IE 9 b 9 QU
Enzyme Modulation/Regulation ● Biochemistry is a process of adaptation ● The body needs a way to slow down and speed up certain reactions ● Since enzymes direct most bodily reactions, enzymes undergo inhibition and activation
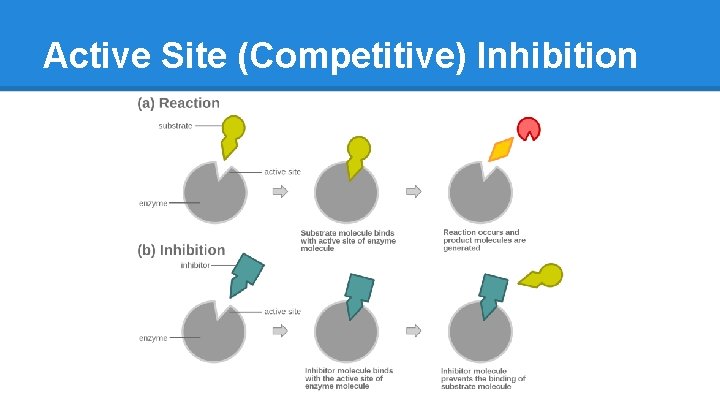
Active Site (Competitive) Inhibition
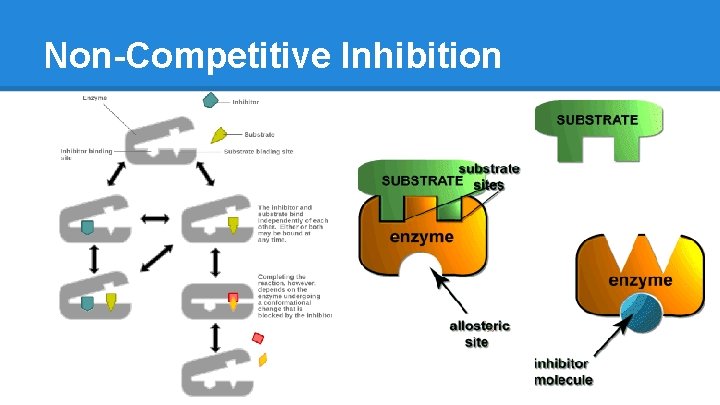
Non-Competitive Inhibition
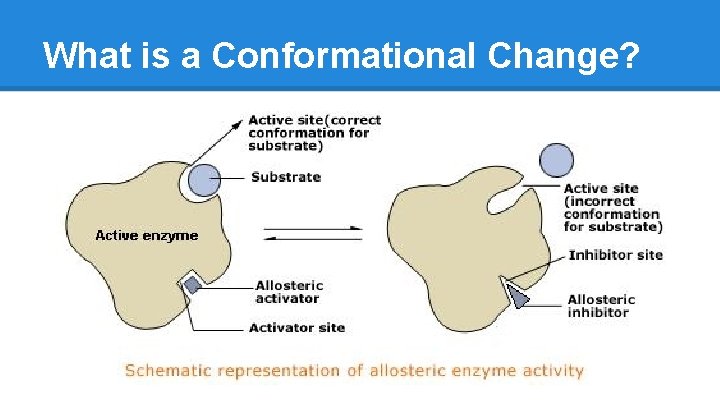
What is a Conformational Change?

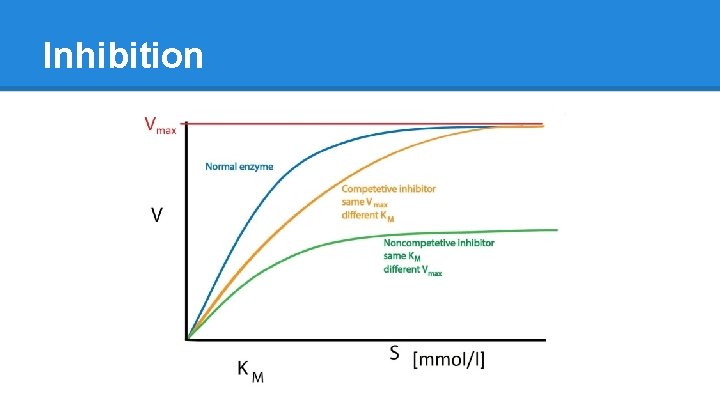
Enzyme Inhibition ● Competitive o Inhibitor is similar to Substrate o Fits into the Active Site but just sits there o Blocks real Substrate from gaining access and inhibits creation of final Product o Reversible ● Non-Competitive o Inhibitor binds to a site other than the active site o Binds to both E and ES complex o Stops enzyme from functioning o Reversible
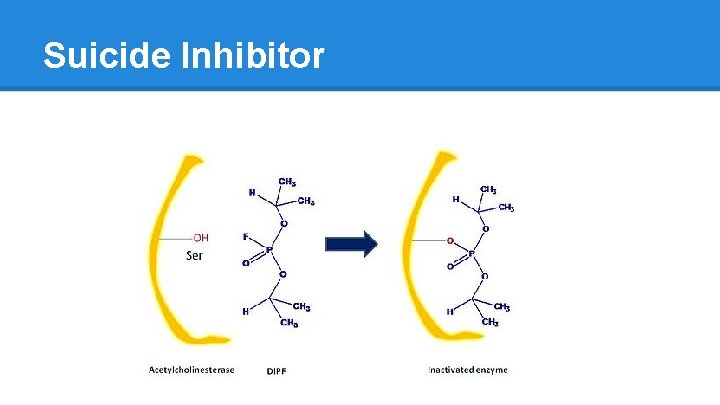
Suicide Inhibitor
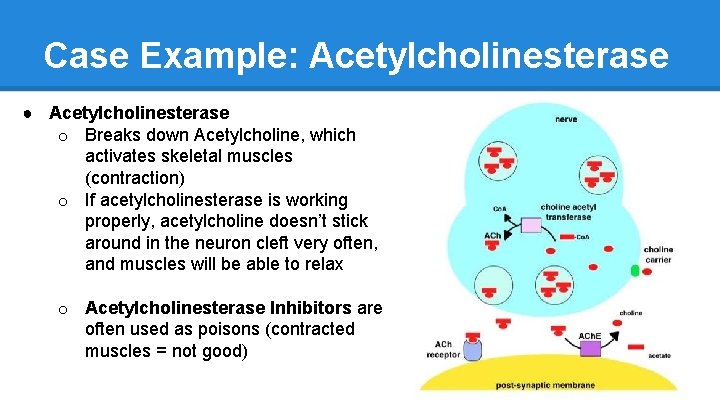
Case Example: Acetylcholinesterase ● Acetylcholinesterase o Breaks down Acetylcholine, which activates skeletal muscles (contraction) o If acetylcholinesterase is working properly, acetylcholine doesn’t stick around in the neuron cleft very often, and muscles will be able to relax o Acetylcholinesterase Inhibitors are often used as poisons (contracted muscles = not good)
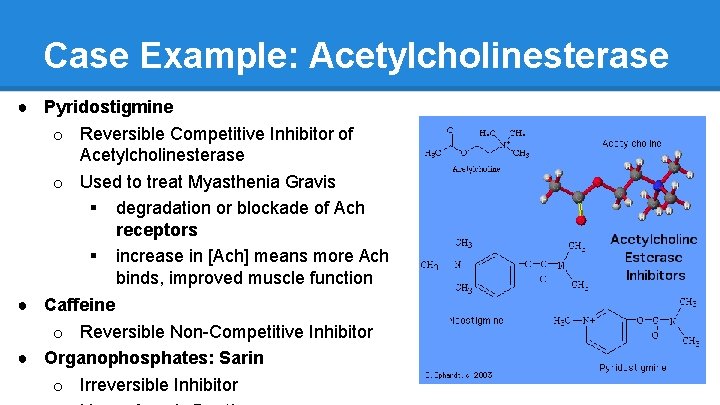
Case Example: Acetylcholinesterase ● Pyridostigmine o Reversible Competitive Inhibitor of Acetylcholinesterase o Used to treat Myasthenia Gravis § degradation or blockade of Ach receptors § increase in [Ach] means more Ach binds, improved muscle function ● Caffeine o Reversible Non-Competitive Inhibitor ● Organophosphates: Sarin o Irreversible Inhibitor
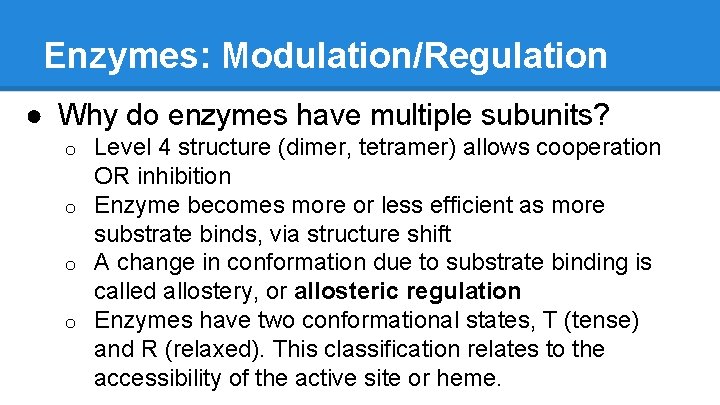
Enzymes: Modulation/Regulation ● Why do enzymes have multiple subunits? Level 4 structure (dimer, tetramer) allows cooperation OR inhibition o Enzyme becomes more or less efficient as more substrate binds, via structure shift o A change in conformation due to substrate binding is called allostery, or allosteric regulation o Enzymes have two conformational states, T (tense) and R (relaxed). This classification relates to the accessibility of the active site or heme. o
Remember Hemoglobin?
Inhibition
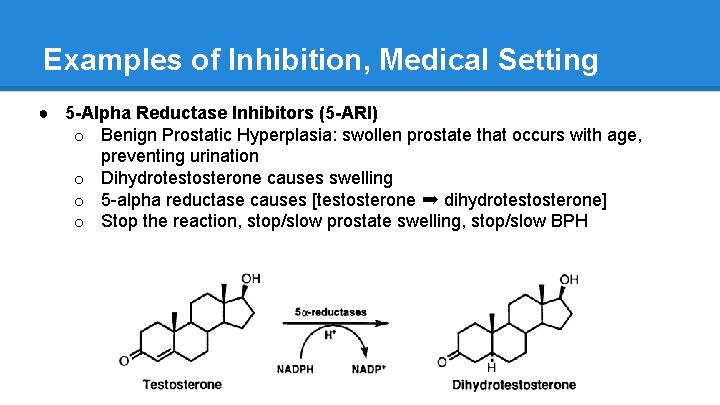
Examples of Inhibition, Medical Setting ● 5 -Alpha Reductase Inhibitors (5 -ARI) o Benign Prostatic Hyperplasia: swollen prostate that occurs with age, preventing urination o Dihydrotestosterone causes swelling o 5 -alpha reductase causes [testosterone ➡ dihydrotestosterone] o Stop the reaction, stop/slow prostate swelling, stop/slow BPH
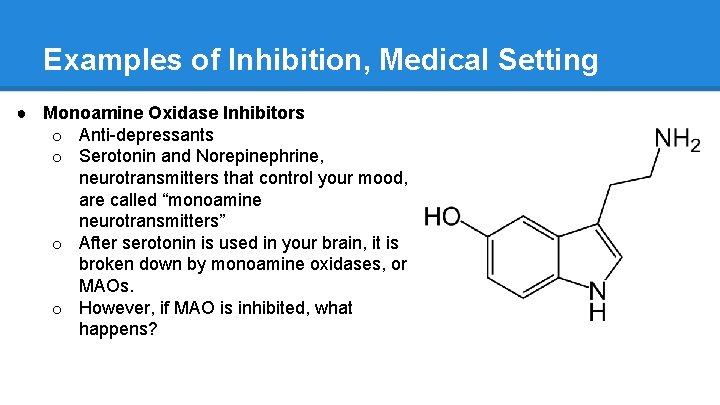
Examples of Inhibition, Medical Setting ● Monoamine Oxidase Inhibitors o Anti-depressants o Serotonin and Norepinephrine, neurotransmitters that control your mood, are called “monoamine neurotransmitters” o After serotonin is used in your brain, it is broken down by monoamine oxidases, or MAOs. o However, if MAO is inhibited, what happens?
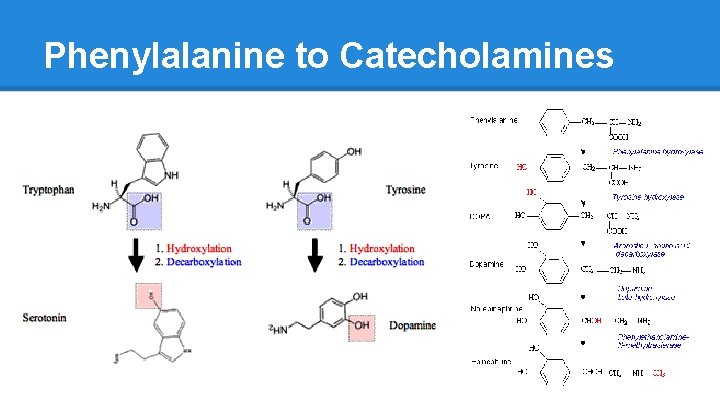
Phenylalanine to Catecholamines

The Key to Competitive Inhibition: Affinity ● Enzymes have very specific substrates ● Substrates fit active sites almost perfectly o Lock and Key model o Determined by number and type of attractive interactions/groups in the active site and on the substrate ● This is described as affinity o How likely is the substrate to bind and stay in the active site? How strong is the attraction? How likely is the substrate to dissociate and drift off again? ● However, the key to making drugs is… o blocking the active site by out-competing the natural substrate (amount) o designing the drug to fit in the active site perfectly (affinity) o Drugs have a higher affinity for the active site than natural substrates and then lock in, preventing the substrate from gaining access

Metabolism: Xenobiotics ● Xenobiotics o drugs, poisons, anything the body doesn’t recognize as a “normal” metabolite ● Foremost defense against xenobiotics is the CYP 450 enzyme family o Pharmacist’s favorite enzyme family o Located (logically) in the liver o Aim: to neutralize foreign, harmful entities and speed excretion ● How does the CYP 450 enzyme family do this? o Phase I: Oxidation, Reduction, Hydrolysis rxns o Phase II: Conjugation Rxns o These reactions make drugs more water-soluable… why?

Thank You for Your Attention! Next class is Carbohydrates and Glycolysis

What I Want You to Know: What is a protein? Give an example of a structural protein and an enzyme. What is the structure of a protein determined by? What are the four levels of the structure of a polypeptide? What is an active site? What types of amino acids are there? What are enzymes? What (generally) do they do? At-a-Glance, how can you pick an enzyme name out of a crowd of scientific jargon? What makes enzymes so special? If a drug is created to block an enzyme, what do the scientists focus on? How many types of enzymes are there? How many types of enzyme inhibition are there and how do they work? What is a conformational change? What is allosteric inhibition/activation?

Sources IMAGES Basic AA Structure: <https: //biochemanics. files. wordpress. com/2013/03/amino-acid-structure. jpg> AA Spreadsheet: <https: //bio 16 mit. files. wordpress. com/2013/07/aminoacids 01. jpg>, Originally Pearson Prentice Hall 2005 Primary AA Structure: <http: //upload. wikimedia. org/wikipedia/commons/thumb/3/38/Protein_primary_structure. svg/2000 px-Protein_primary_structure. svg. png> Tiara: <http: //1. bp. blogspot. com/-0 Gbj. OAVm. C 2 U/Th. Noel. Vw. Hs. I/AAAAO 8 c/PJKEq. A 7 h. Dvc/s 1600/Cambridge+Lover%2527 s+Knot+tiara. JPG> Four Stages of Protein Folding: <http: //cnx. org/resources/fb 4997 cac 700 d 686322 c 9930 cf 9 abc 5 cfa 187982/Figure_03_04_09. jpg> Hemoglobin image: <http: //bioserv. fiu. edu/~walterm/B/biomolecomplete/lectur 35. jpg> Hemoglobin vs Myoglobin information: <http: //www. wiley. com/college/boyer/0470003790/structure/Hb. Mb/Eval. Text. html> Hemoglobin vs Myoglobin: <http: //m. harunyahya. com/tr/Buku/4583/The-Miracle-Of-The-Blood-And-Heart/chapter/5015/Blood-The-incomparable-liquid-of-life--5 -> Active Site Image: <http: //andromeda. rutgers. edu/~huskey/images/chymo_active_site 1. jpg> Heme Image: <http: //www. buzzle. com/images/diagrams/hemoglobin-structure. jpg> Collagen: <http: //world. gold-collagen. com/wp-content/uploads/2011/11/mechanism 2 -large. jpg> Enzyme image 1: http: //media 1. shmoop. com/images/biology/biobook_flow_5 a. png Hemoglobin Allostery: http: //www. biochem. arizona. edu/classes/bioc 460/spring/460 web/lectures/LEC 8 -9_Mb. Hb/Stry 6 Fig 7 -11 Concerted. Model. jpg Enzyme Transition State Graph: https: //biochemistry 3 rst. files. wordpress. com/2014/03/activation-energy-diagram. jpg

Sources, Cont Competitive Inhibition Image: http: //upload. wikimedia. org/wikipedia/commons/thumb/f/fb/Comp_inhib. svg/2000 px-Comp_inhib. svg. png Non-Competitive Image: http: //mandevillehigh. stpsb. org/teachersites/laura_decker/ap_enzyme_notes_files/image 005. gif Suicide Inhibitor: http: //upload. wikimedia. org/wikipedia/commons/thumb/2/21/Group_specific_reagent. jpg/550 px-Group_specific_reagent. jpg Acetylecholinesterase: http: //upload. wikimedia. org/wikipedia/commons/8/89/AChe_mechanism_of_action. jpg Ac. E Inhibitors: http: //elmhcx 9. elmhurst. edu/~chm/vchembook/images 2/662 esteraseinhibit. gif Enzyme Allostery: http: //images. tutorvista. com/content/cellular-macromolecules/allosteric-inhibition-process. jpeg Metabolic Poster: http: //www. sciencemusings. com/blog/uploaded_images/Metabolism-733633. jpg Enzyme Inhibition Graph: http: //fblt. cz/wp-content/uploads/2013/12/graf-inhibice-ENG-01. jpg Testosterone to DHT: http: //patentimages. storage. googleapis. com/EP 0719278 B 1/0001. png Serotonin: http: //upload. wikimedia. org/wikipedia/commons/d/dd/Serotonin-skeletal. png Tyrosine to Catecholamines: http: //www. pharmacorama. com/en/Sections/images/Catecholamines_3_clip_image 002. gif Serotonin from Tyrosine: http: //www. scq. ubc. ca/wp-content/uploads/2007/05/banana. gif
- Slides: 54