Basic Chemistry Section 2 1 What is an

Basic Chemistry Section 2 -1

What is an atom? § The basic unit of matter.
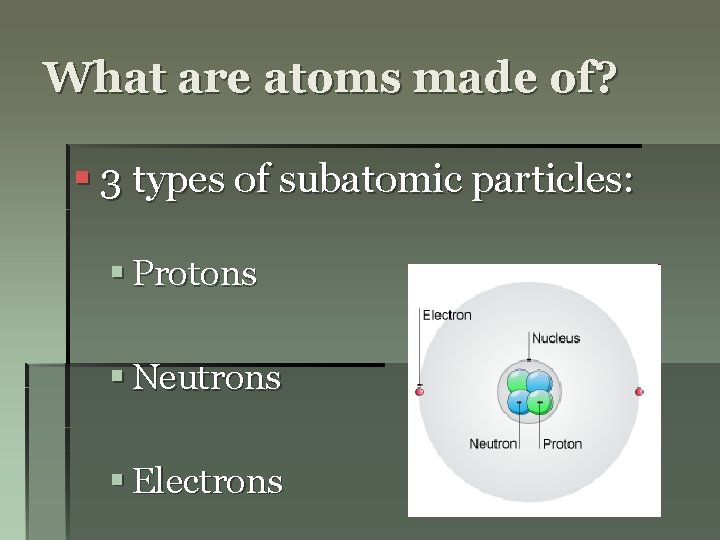
What are atoms made of? § 3 types of subatomic particles: § Protons § Neutrons § Electrons
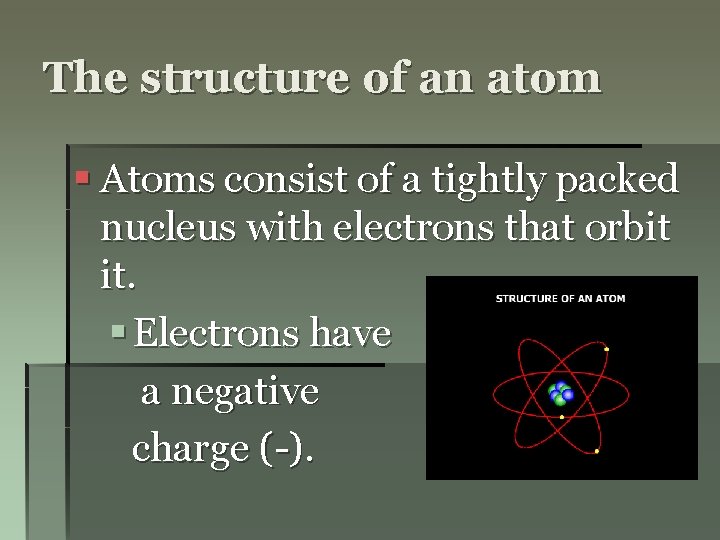
The structure of an atom § Atoms consist of a tightly packed nucleus with electrons that orbit it. § Electrons have a negative charge (-).

Electron Configuration §Electrons are found in various shells or clouds that orbit the nucleus §Each shell can hold only a certain number of electrons § 1 st shell = 2 electrons § 2 nd shell = 8 electrons

Valence electrons § The electrons found in the outermost shell are called valence electrons
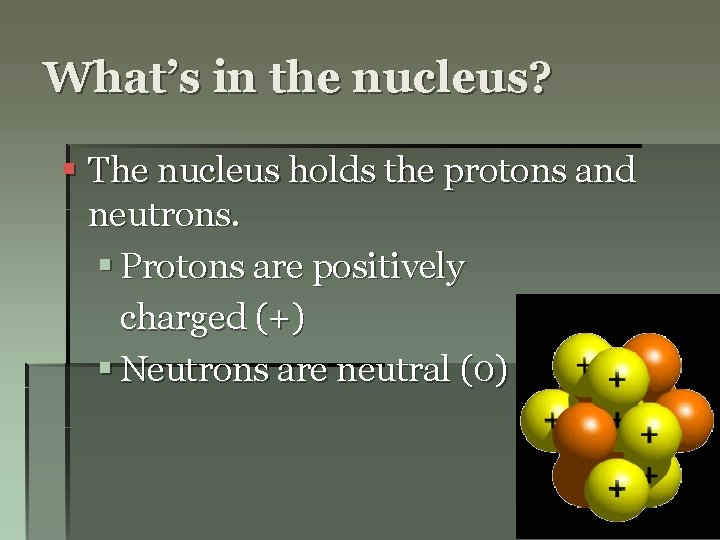
What’s in the nucleus? § The nucleus holds the protons and neutrons. § Protons are positively charged (+) § Neutrons are neutral (0)
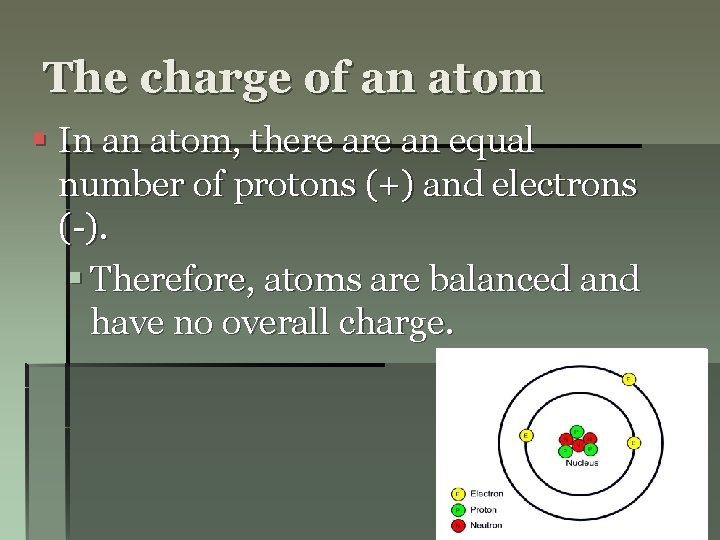
The charge of an atom § In an atom, there an equal number of protons (+) and electrons (-). § Therefore, atoms are balanced and have no overall charge.

Element § A chemical substance that’s made of only one type of atom. § There are over 100 types § i. e. pure gold, carbon, hydrogen, etc.

6 Essential Elements for Life § There are 6 elements that are required for living organisms § Carbon (C) § Hydrogen (H) § Oxygen (O) § Nitrogen (N) § Phosphorus (P) § Sulfur (S)

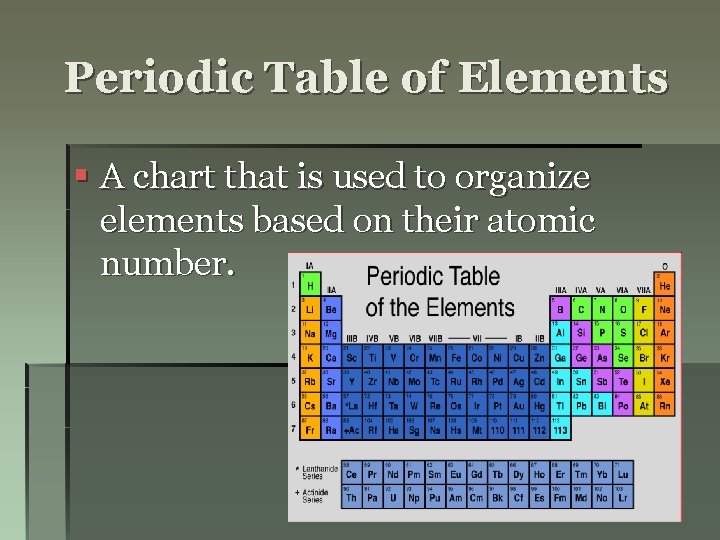
Periodic Table of Elements § A chart that is used to organize elements based on their atomic number.

Atomic number (Z) § Shows the number of protons in each atom § Each element has its own unique atomic number. § Ex: 79 protons = Gold 6 protons = Carbon
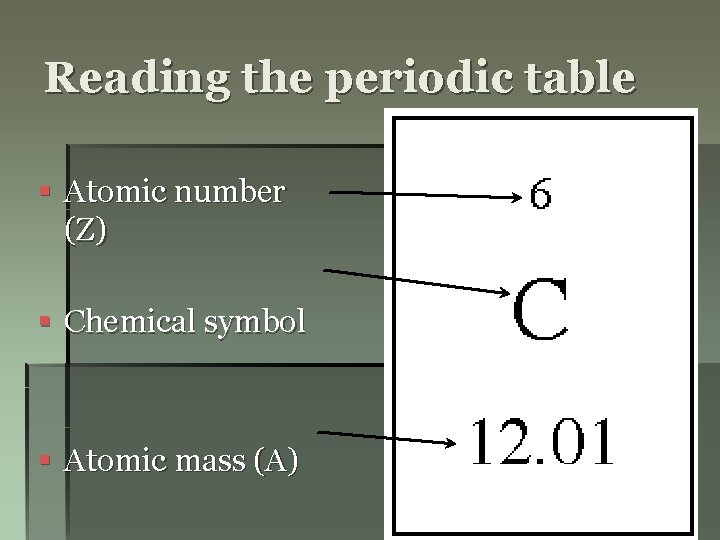
Reading the periodic table § Atomic number (Z) § Chemical symbol § Atomic mass (A)
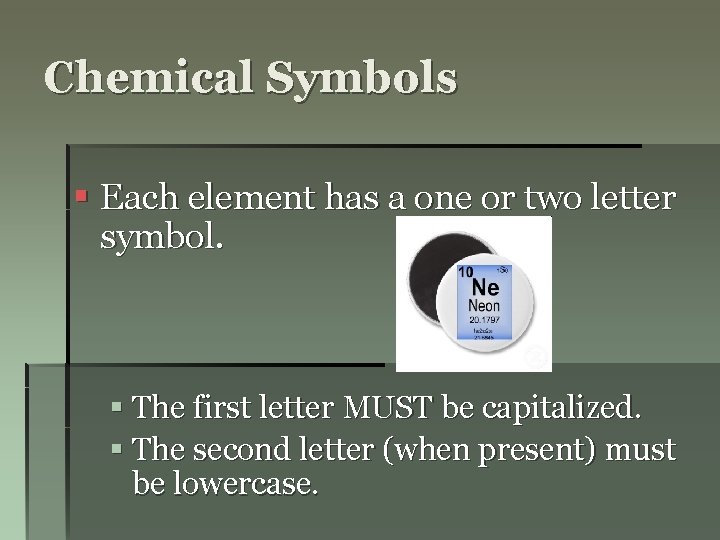
Chemical Symbols § Each element has a one or two letter symbol. § The first letter MUST be capitalized. § The second letter (when present) must be lowercase.

Atomic Mass (A) § Shows the average mass of the atom § Measured in a. m. u. (atomic mass units) § Each proton weighs one a. m. u. § Each neutron weighs one a. m. u. § Electrons are so small they do not affect the mass
§ In other words, the atomic mass tells us how many protons and neutrons are in the nucleus. § #of Neutrons = Atomic Mass (A) - #of Protons
Chemical Bonds
Chemical Bonds § There are 4 major types of bonds. § Hold atoms together to form molecules § Store energy § Involve valence electrons

Compound § A substance formed by the chemical combination of two or more elements. § Ex: § H 2 O – water § Na. Cl - Salt

1) Van der Waals Force (Extremely weak) § Created when normal movement of electrons causes temporary charges and attraction.
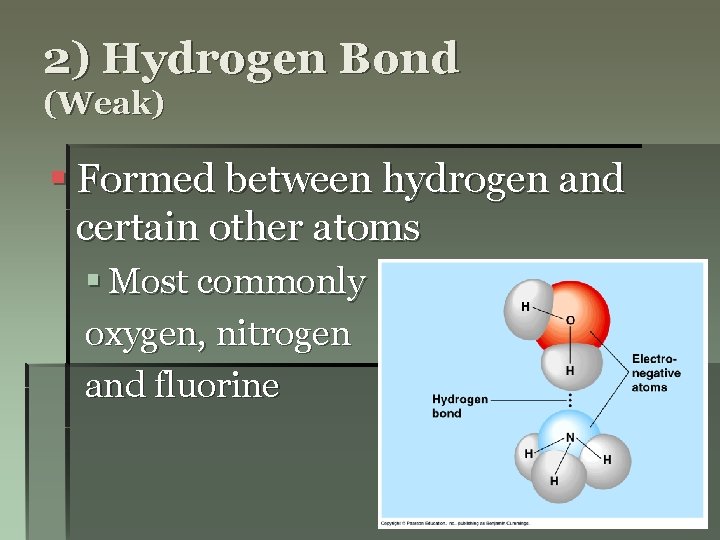
2) Hydrogen Bond (Weak) § Formed between hydrogen and certain other atoms § Most commonly oxygen, nitrogen and fluorine

3) Ionic Bond (Medium) § Created when one atom “steals” an electron from another atom, thus creating ions (one positive, one negative). § The attraction between these oppositely charged ions forms the bond
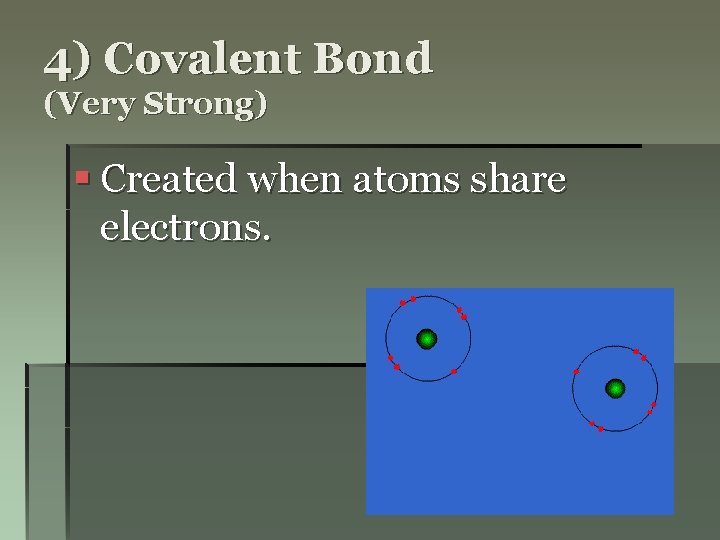
4) Covalent Bond (Very Strong) § Created when atoms share electrons.

All bonds store chemical energy!
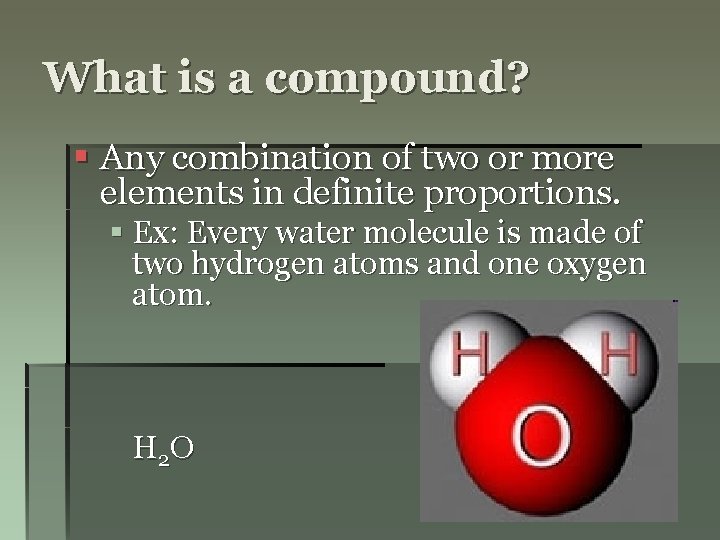
What is a compound? § Any combination of two or more elements in definite proportions. § Ex: Every water molecule is made of two hydrogen atoms and one oxygen atom. H 2 O
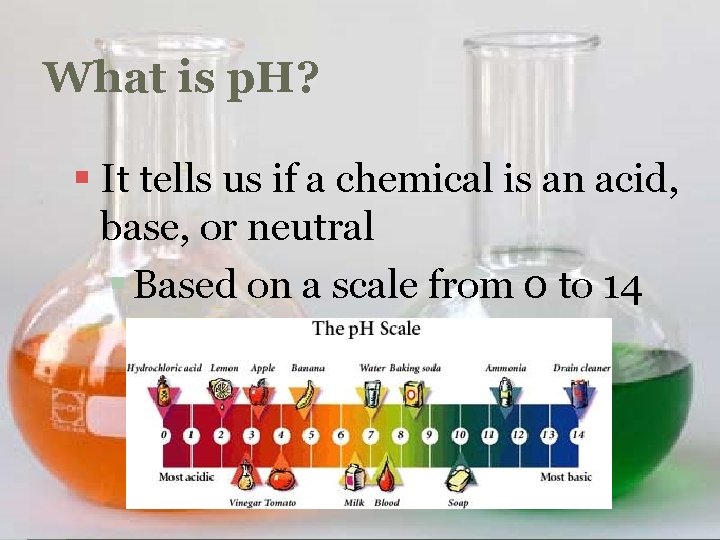
What is p. H? § It tells us if a chemical is an acid, base, or neutral § Based on a scale from 0 to 14

Acid § Any chemical with a p. H below 7 § Releases hydrogen (H+) ions in water

Base § Any chemical with a p. H above 7 § Releases hydroxide (OH-) ions in water

Neutral § Any chemical with a p. H of exactly 7 § Neither an acid nor a base § Ex: Water

Buffer § A chemical that keeps the p. H from changing significantly.
- Slides: 31