Basic Chemistry Organic Chemistry and Water Section 1

Basic Chemistry, Organic Chemistry, and Water Section 1 -2
Organization of Matter

Atoms and Subatomic Particles • Protons- positive charge; located in nucleus • Neutrons- no charge; located in nucleus • Electrons- negative charge; located outside the nucleus in energy clouds (energy levels, electron shells
Atoms and Subatomic Particles • Atomic Number- equal to the number of protons an atom contains; the one thing that makes an atom unique • Atomic Mass- equal to the number of protons + the number of neutrons • Example: Flourine
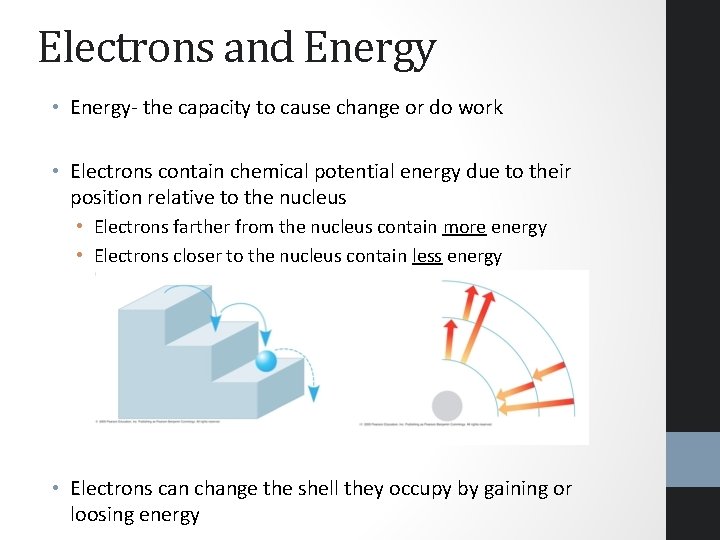
Electrons and Energy • Energy- the capacity to cause change or do work • Electrons contain chemical potential energy due to their position relative to the nucleus • Electrons farther from the nucleus contain more energy • Electrons closer to the nucleus contain less energy • Electrons can change the shell they occupy by gaining or loosing energy

Chemical Compounds and Types of Bonds

Chemical Bonding • Chemical bonding is controlled by two factors: • Valence Electrons- outmost electrons of an atom • Electronegativity- the attraction an atom has for its electrons and the electrons of other atoms • The more valence electrons an atom has and the closer those electrons are to the nucleus (the lower the shell) the more electronegative that atom is
Covalent Bonds • Result from a sharing of electrons • Molecule- two or more atoms held together by a covalent bond • Two atoms can be bonded together by one or multiple pairs of electrons • Single bonds- sharing two electrons • Double bonds- sharing four electrons • Triple bonds- sharing six electrons
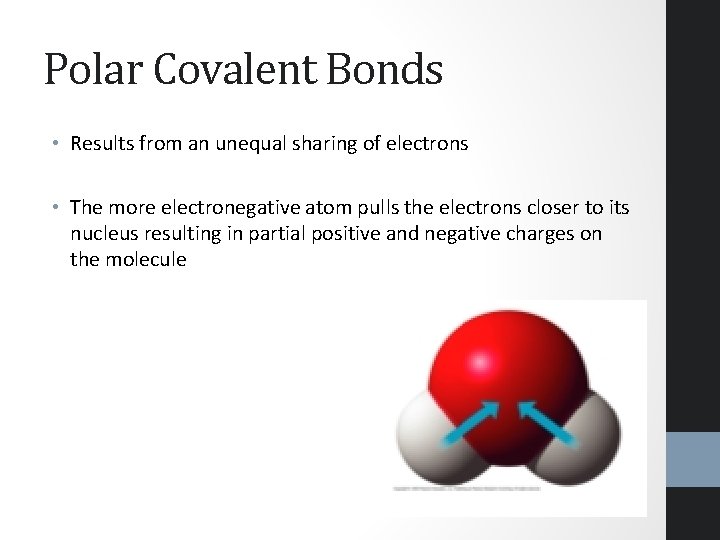
Polar Covalent Bonds • Results from an unequal sharing of electrons • The more electronegative atom pulls the electrons closer to its nucleus resulting in partial positive and negative charges on the molecule
Ionic Bonds • Result from a complete transfer of electrons • Electrons are transferred from the less electronegative atom to the more electronegative atom resulting in two charged particles that then form an attraction to each other

Weak Chemical “Bonds”
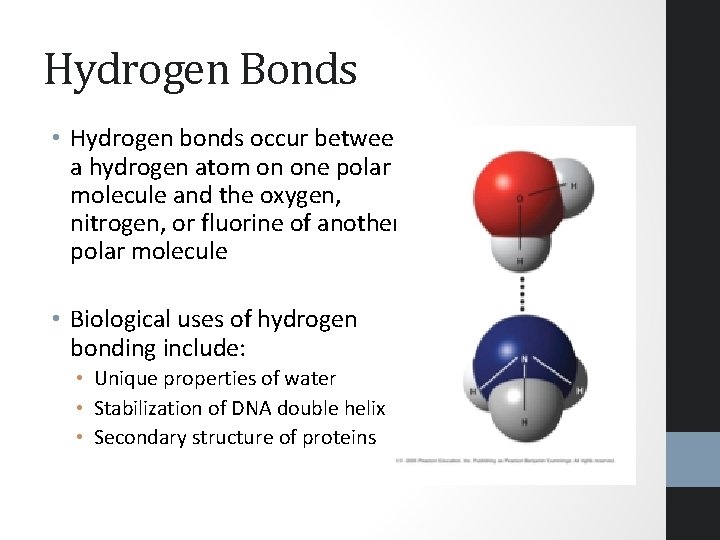
Hydrogen Bonds • Hydrogen bonds occur between a hydrogen atom on one polar molecule and the oxygen, nitrogen, or fluorine of another polar molecule • Biological uses of hydrogen bonding include: • Unique properties of water • Stabilization of DNA double helix • Secondary structure of proteins

Van der Walls Interactions • Van der Walls interactions are weak interactions that occur only when atoms, molecules, or regions of large molecules are very close together • Important in the folding and stabilization of large molecules such as proteins and nucleic acids

Chemical Structure of Water
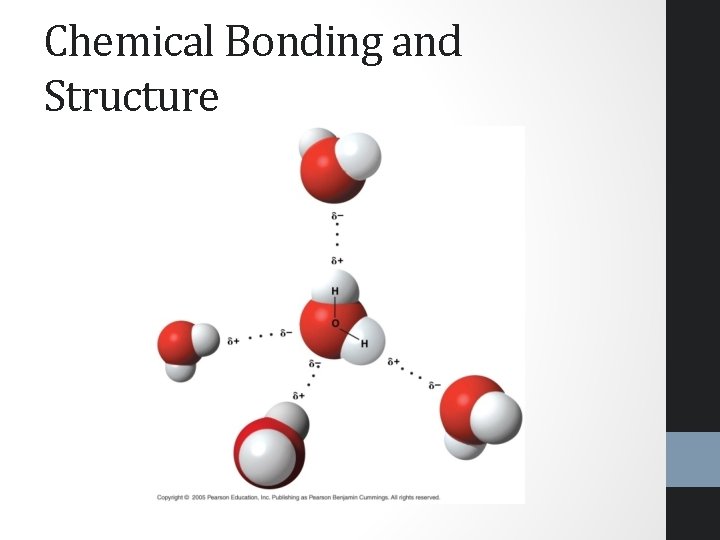
Chemical Bonding and Structure

Chemical Bonding and Structure • Water is a polar molecule • Oxygen is highly electronegative- it has a large attraction for electrons and pulls them closer • This creates two polar bonds within the water molecule • Water has an overall bent shape • The oxygen portion of the atom has a partially negative charge • The hydrogen end of the atom has a partially positive charge • Because of hydrogen bonding, water is more structured than most liquids
Properties of Water

Cohesive Behavior

Cohesion • The ability of water to stick to other water molecules • Cohesion is possible because of the hydrogen bonds between water molecules, which is possible because of the polarity of the water molecules

Adhesion • The ability of water molecules to stick to other things • Adhesion is also possible because of the polarity of water molecules; the charges created by the bond polarity causes it to have electrostatic attractions to other partially charged surfaces

Surface Tension • A measure of how difficult it is to stretch or break the surface of a liquid • Water has a high surface tension because of the hydrogen bonding between molecules

Application of Cohesive Behavior

Temperature Moderation

Heat vs. Temperature • Heat is the total amount of kinetic energy inside a substance • Temperature is the average kinetic energy of the molecules • Heat always passes from warmer objects to colder objects • Heat must be absorbed to increase temperature

Specific Heat • Water has a very high specific heat; it takes a lot of heat energy to increase the temperature of water • Before the molecules can move faster (increase the average kinetic energy, or temperature) the hydrogen bonds must be disrupted • So the first energy that is applied to water must be used to disrupt hydrogen bonds

Evaporative Cooling • Water has a high heat of vaporization- the amount of heat necessary to convert water from liquid to a gas • Because of this, the molecules at the surface of a liquid are the “hottest” or contain the greatest amount of energy • As molecules leave the surface of the liquid, those just below the surface are cooler (contain less energy)

Applications of Temperature Moderation • The water that covers the earth’s surface helps to regulate the global climate to a range that is suitable for life by absorbing excess heat energy • The water inside of organisms helps them to resist drastic changes in temperature • Water evaporating from the surface of bodies of water keeps them in a temperature range that allows organisms living within them to survive • Water evaporating off the surface of terrestrial organisms helps them to regulate their body temperatures

Expansion Upon Freezing • Water expands and becomes less dense as it freezes • The hydrogen bonds allow the water to form a crystalline structure where each water molecule is as far away from others as possible • The surface of bodies of water, as heat is lost freeze and float on top, insulating the water below so that it may still sustain life
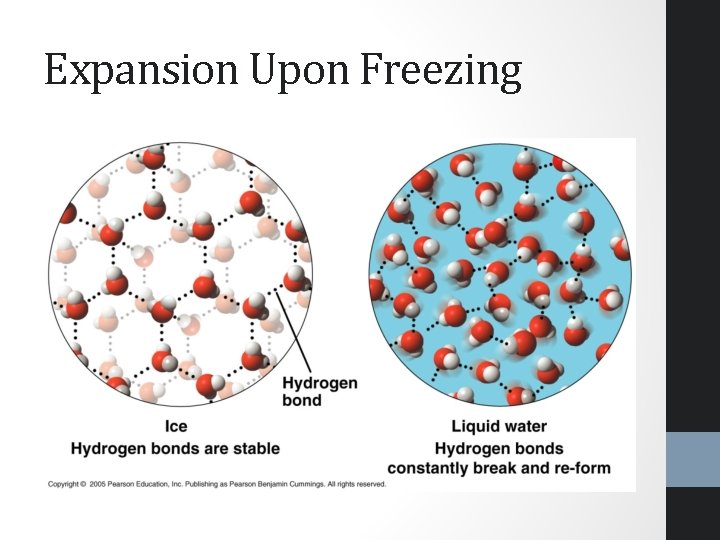
Expansion Upon Freezing

Solvation Behavior

Water is the Solvent of Life • It is capable of dissolving ionic compounds and polar covalent compounds • Water molecules completely surround each solute particle
Concentration • Molarity is the measure of the concentration of a solution • Molarity= moles of solute/L of solution

Hydrophobic vs. Hydrophyllic

Hydrophillic • Any substance that has an affinity for water • Not the same thing as soluble • Large molecules that have polar regions can be hydrophyllic without actually dissolving

Hydrophobic • Any substance that repels water • Substances with mostly nonpolar bonds are hydrophobic

Hydrophobic vs. Hydrophyllic ***** It does not have to be an entire molecule that is hydrophobic or hydrophyllic- regions of molecules can be hydrophobic or hydrophyllic!!!!! *****

Acids, Bases, and p. H

Acids • Any substance that increase the hydrogen ion concentration of a solution • Acidic solutions have more hydrogen ions than hydroxide ions

Bases • Any substance that reduces the hydrogen ion concentration of a solution • Accepts hydrogen ions • Increase the number of hydroxide ions
p. H • The p. H scale is a way of measuring how acidic or basic a substance is • Biological processes have very specific ranges of p. H in which they can function at optimal levels • As the hydrogen ion concentration increases, the p. H decreases • Each difference on the p. H scale corresponds to a tenfold hydrogen ion difference • When the p. H changes slightly, the H+ concentration changes dramatically; by a factor of 10 for every step of the p. H scale
- Slides: 40