Basic Chemistry calculations Topic 01 Asiri Foundation R
Basic Chemistry calculations Topic 01 Asiri Foundation R. Satheesh B. Eng. (Hons) Lecturer – Biomedical Science International Institute of Health Sciences

Objective q Molar mass q Number of moles q Number of atoms in samples q Concentration of solutions q Empirical formulas q Avogadro constant

Carrying out Calculations In chemistry, must deal with several mathematical functions. Scientific Notation • • Makes it easier to deal with large numbers, especially concentrations Written as A × 10 b, where A is a decimal number and b is a whole number Example: Avogadro’s number 602 213 670 000 000 000 It is very inconvenient to write this. Instead, use scientific notation: 6. 022 × 1023
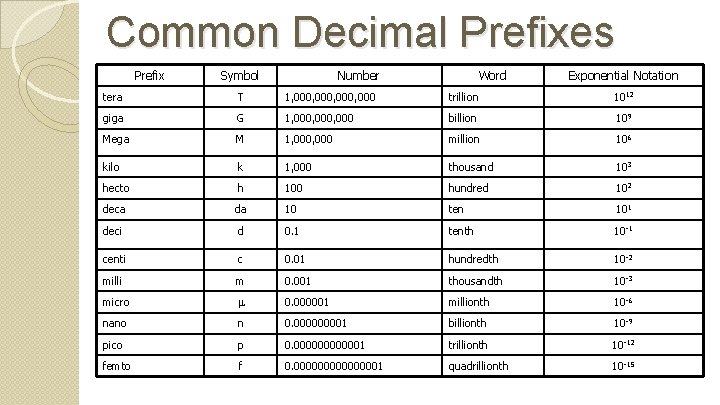
Common Decimal Prefixes Prefix Symbol Number Word Exponential Notation tera T 1, 000, 000 trillion 1012 giga G 1, 000, 000 billion 109 Mega M 1, 000 million 106 kilo k 1, 000 thousand 103 hecto h 100 hundred 102 deca da 10 ten 101 deci d 0. 1 tenth 10 -1 centi c 0. 01 hundredth 10 -2 milli m 0. 001 thousandth 10 -3 micro 0. 000001 millionth 10 -6 nano n 0. 00001 billionth 10 -9 pico p 0. 0000001 trillionth 10 -12 femto f 0. 00000001 quadrillionth 10 -15

STOICHIOMETRY The study of the quantitative aspects of chemical reactions.

The moles � Mole: SI unit of the amount of a substance Definition: A mole is the number of atoms in exactly 12 g of the carbon-12 isotope This number is called Avogadro’s number and is given by 6. 022 × 1023 The mole is NOT just a counting unit, like the dozen, which specifies only the number of objects. The definition of a mole specifies the number of objects in a fixed mass of substance. Mass spectrometry tells us that the mass of a carbon-12 atom is 1. 9926× 10 -23 g. No. of carbon-12 atoms = = atomic mass (g) mass of one atom (g) 12 g _ 1. 9926× 10 -23 g = 6. 022 × 1023 atoms

� � � One mole contains Avogadro’s Number (6. 022 x 1023) A mole is the amount of a substance of a system which contains as many elementary entities as there atoms in 0. 012 kg (or 12 g) of Carbon-12 A mole is that quantity of a substance whose mass in grams is the same as its formula weight E. g. Fe 55. 85 Iron has an atomic mass or 55. 85 gmol-1, so one mole of iron has a mass or 55. 85 g
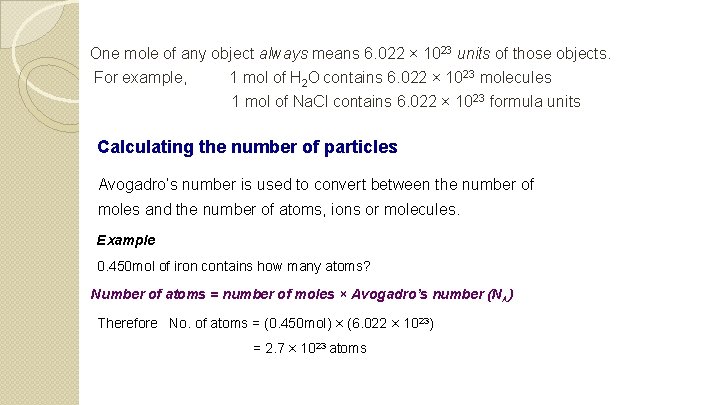
One mole of any object always means 6. 022 × 1023 units of those objects. For example, 1 mol of H 2 O contains 6. 022 × 1023 molecules 1 mol of Na. Cl contains 6. 022 × 10 23 formula units Calculating the number of particles Avogadro’s number is used to convert between the number of moles and the number of atoms, ions or molecules. Example 0. 450 mol of iron contains how many atoms? Number of atoms = number of moles × Avogadro’s number (NA) Therefore No. of atoms = (0. 450 mol) × (6. 022 × 1023) = 2. 7 × 1023 atoms
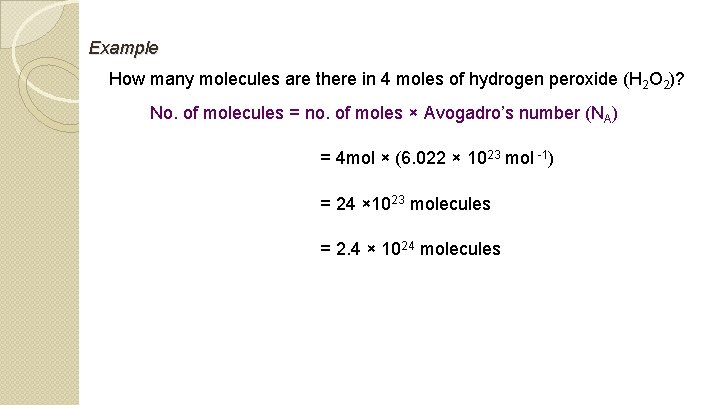
Example How many molecules are there in 4 moles of hydrogen peroxide (H 2 O 2)? No. of molecules = no. of moles × Avogadro’s number (NA) = 4 mol × (6. 022 × 1023 mol -1) = 24 × 1023 molecules = 2. 4 × 1024 molecules

Calculating the mass of one molecule Example: What is the mass of one molecule of water? Step 1: Calculate the molar mass of water Molar mass of water = (2 × atomic mass H) + (1 × atomic mass O) Molar mass H 2 O = (2 × 1. 008 g mol-1) + (1 × 16. 000 g mol-1) = 18. 00 gmol-1 Step 2: Employ Avogadro’s number Mass of one molecule = Molar mass Avogadro’s no. = 18. 00 g mol-1 6. 022× 1023 mol-1 k = 2. 992× 10 -23 g Note: Always check the units you have in your answer to ensure you are correct
![Example Calculate the mass of one molecule of ammonium carbonate [(NH 4)2 CO 3] Example Calculate the mass of one molecule of ammonium carbonate [(NH 4)2 CO 3]](http://slidetodoc.com/presentation_image_h2/7007792c16aa19af6df28212ec789746/image-11.jpg)
Example Calculate the mass of one molecule of ammonium carbonate [(NH 4)2 CO 3] Step 1: Calculate the molar mass 2 Nitrogen atoms 2 × 14. 01 gmol-1 = 28. 02 gmol-1 8 Hydrogen atoms 8 × 1. 008 gmol-1 = 8. 064 gmol-1 1 Carbon atom 1 × 12. 01 gmol-1 = 12. 01 gmol-1 3 Oxygen atoms 3 × 16. 00 gmol-1 = 48. 00 gmol-1 Total = 96. 09 gmol-1 Step 2: Employ Avogadro’s Number, NA Mass of one molecule = 96. 09 gmol-1 . 6. 022× 1023 mol-1 = 1. 59 × 10 -22 g
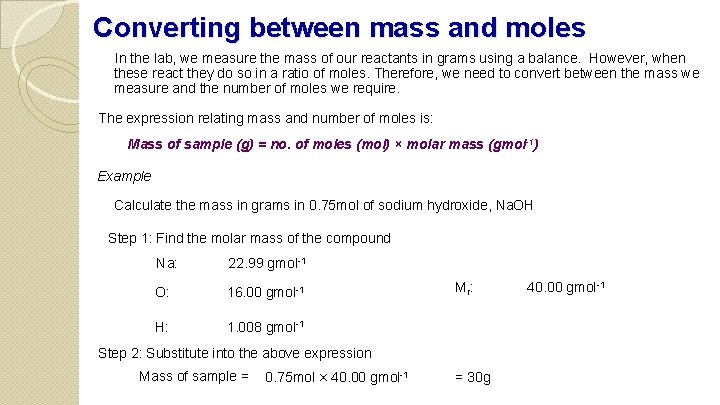
Converting between mass and moles In the lab, we measure the mass of our reactants in grams using a balance. However, when these react they do so in a ratio of moles. Therefore, we need to convert between the mass we measure and the number of moles we require. The expression relating mass and number of moles is: Mass of sample (g) = no. of moles (mol) × molar mass (gmol-1) Example Calculate the mass in grams in 0. 75 mol of sodium hydroxide, Na. OH Step 1: Find the molar mass of the compound Na: 22. 99 gmol-1 O: 16. 00 gmol-1 H: 1. 008 gmol-1 Mr : Step 2: Substitute into the above expression Mass of sample = 0. 75 mol × 40. 00 gmol-1 = 30 g 40. 00 gmol-1
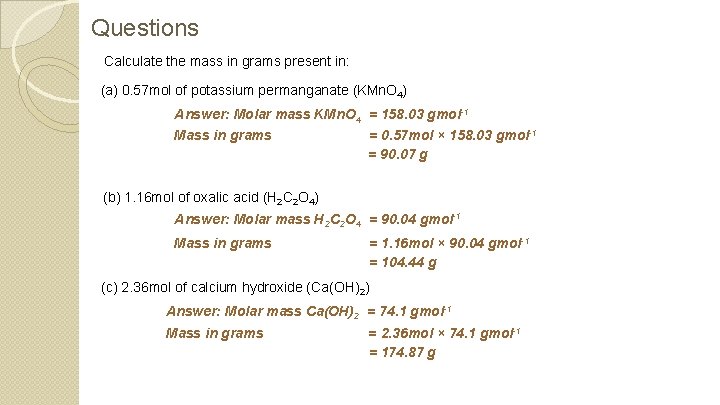
Questions Calculate the mass in grams present in: (a) 0. 57 mol of potassium permanganate (KMn. O 4) Answer: Molar mass KMn. O 4 = 158. 03 gmol-1 Mass in grams = 0. 57 mol × 158. 03 gmol-1 = 90. 07 g (b) 1. 16 mol of oxalic acid (H 2 C 2 O 4) Answer: Molar mass H 2 C 2 O 4 = 90. 04 gmol-1 Mass in grams = 1. 16 mol × 90. 04 gmol-1 = 104. 44 g (c) 2. 36 mol of calcium hydroxide (Ca(OH)2) Answer: Molar mass Ca(OH)2 = 74. 1 gmol-1 Mass in grams = 2. 36 mol × 74. 1 gmol-1 = 174. 87 g

Molarity Some chemical reactions involve aqueous solutions of reactants The concentration of a solution is the amount of solute present in a given quantity of solvent or solution This concentration may be expressed in terms of molarity (M) or molar concentration: M = Molarity = no. of moles volume in Litres Molarity is the number of moles of solute in 1 Litre (L) of solution
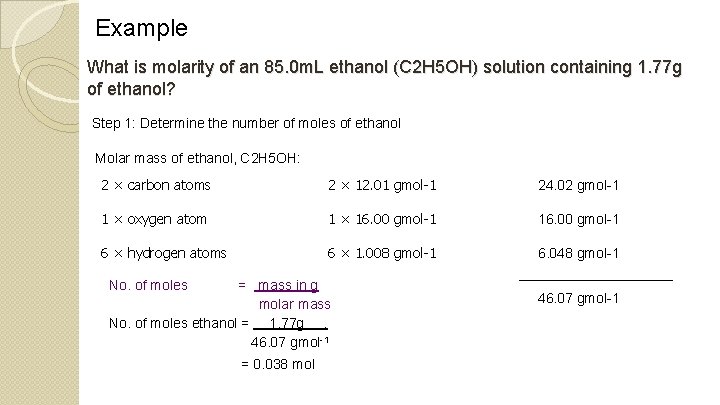
Example What is molarity of an 85. 0 m. L ethanol (C 2 H 5 OH) solution containing 1. 77 g of ethanol? Step 1: Determine the number of moles of ethanol Molar mass of ethanol, C 2 H 5 OH: 2 × carbon atoms 2 × 12. 01 gmol-1 24. 02 gmol-1 1 × oxygen atom 1 × 16. 00 gmol-1 6 × hydrogen atoms 6 × 1. 008 gmol-1 6. 048 gmol-1 No. of moles = mass in g molar mass No. of moles ethanol = 1. 77 g. 46. 07 gmol-1 = 0. 038 mol 46. 07 gmol-1
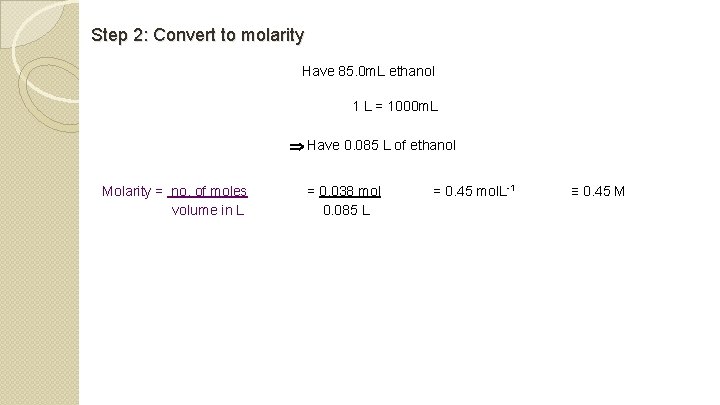
Step 2: Convert to molarity Have 85. 0 m. L ethanol 1 L = 1000 m. L Have 0. 085 L of ethanol Molarity = no. of moles volume in L = 0. 038 mol 0. 085 L = 0. 45 mol. L-1 ≡ 0. 45 M
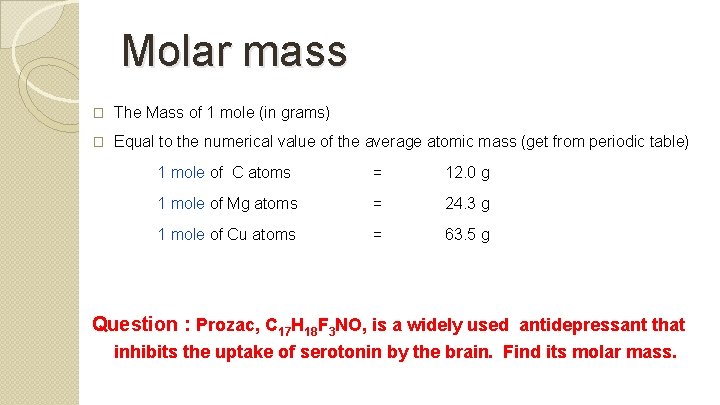
Molar mass � The Mass of 1 mole (in grams) � Equal to the numerical value of the average atomic mass (get from periodic table) 1 mole of C atoms = 12. 0 g 1 mole of Mg atoms = 24. 3 g 1 mole of Cu atoms = 63. 5 g Question : Prozac, C 17 H 18 F 3 NO, is a widely used antidepressant that inhibits the uptake of serotonin by the brain. Find its molar mass.

Concentration of solutions
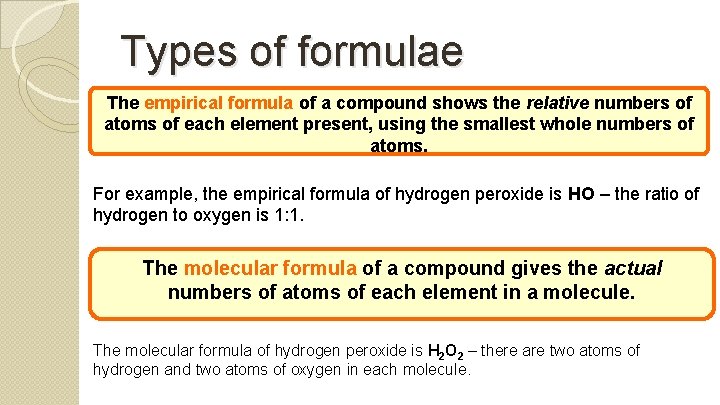
Types of formulae The empirical formula of a compound shows the relative numbers of atoms of each element present, using the smallest whole numbers of atoms. For example, the empirical formula of hydrogen peroxide is HO – the ratio of hydrogen to oxygen is 1: 1. The molecular formula of a compound gives the actual numbers of atoms of each element in a molecule. The molecular formula of hydrogen peroxide is H 2 O 2 – there are two atoms of hydrogen and two atoms of oxygen in each molecule.
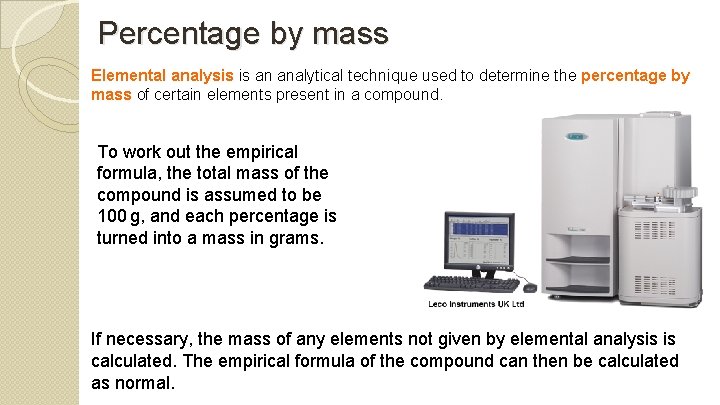
Percentage by mass Elemental analysis is an analytical technique used to determine the percentage by mass of certain elements present in a compound. To work out the empirical formula, the total mass of the compound is assumed to be 100 g, and each percentage is turned into a mass in grams. If necessary, the mass of any elements not given by elemental analysis is calculated. The empirical formula of the compound can then be calculated as normal.
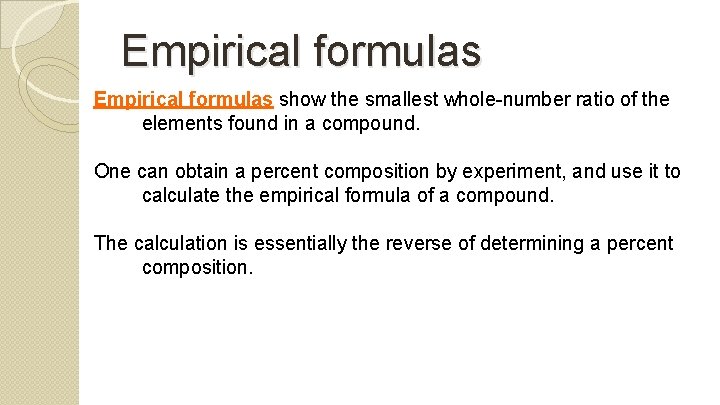
Empirical formulas show the smallest whole-number ratio of the elements found in a compound. One can obtain a percent composition by experiment, and use it to calculate the empirical formula of a compound. The calculation is essentially the reverse of determining a percent composition.

Steps: (a) assume 100. 00 g of compound, so mass percent of each element can be expressed in grams (b) calculate number of moles of each element present in that mass (c) determine mole ratios of elements (d) write empirical formula
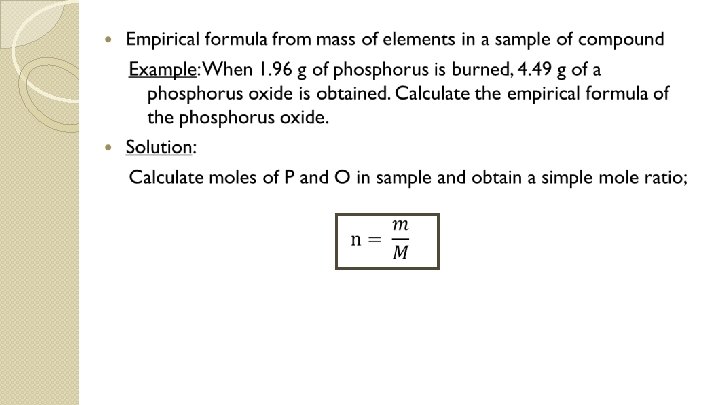
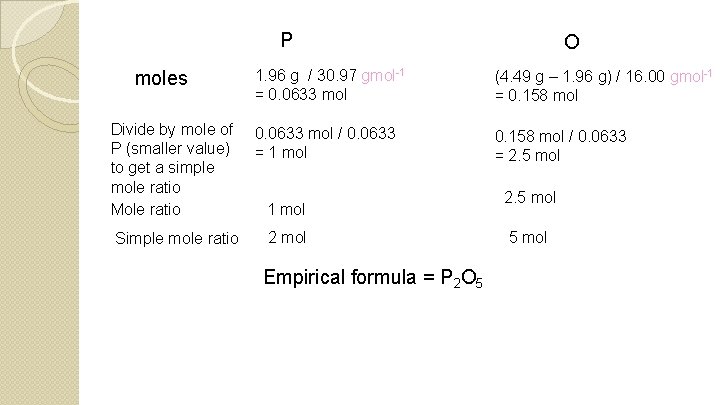
P moles Divide by mole of P (smaller value) to get a simple mole ratio Mole ratio Simple mole ratio O 1. 96 g / 30. 97 gmol-1 = 0. 0633 mol (4. 49 g – 1. 96 g) / 16. 00 gmol-1 = 0. 158 mol 0. 0633 mol / 0. 0633 = 1 mol 0. 158 mol / 0. 0633 = 2. 5 mol 1 mol 2 mol Empirical formula = P 2 O 5 2. 5 mol
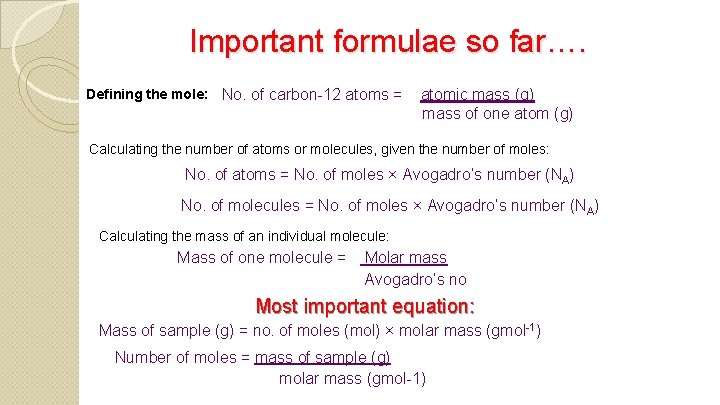
Important formulae so far…. Defining the mole: No. of carbon-12 atoms = atomic mass (g) mass of one atom (g) Calculating the number of atoms or molecules, given the number of moles: No. of atoms = No. of moles × Avogadro’s number (NA) No. of molecules = No. of moles × Avogadro’s number (NA) Calculating the mass of an individual molecule: Mass of one molecule = Molar mass Avogadro’s no Most important equation: Mass of sample (g) = no. of moles (mol) × molar mass (gmol-1) Number of moles = mass of sample (g) molar mass (gmol-1)
- Slides: 25