Basic Chemistry But this is Biology atoms elements
Basic Chemistry …. But this is Biology? ? ? atoms, elements, molecules, compounds
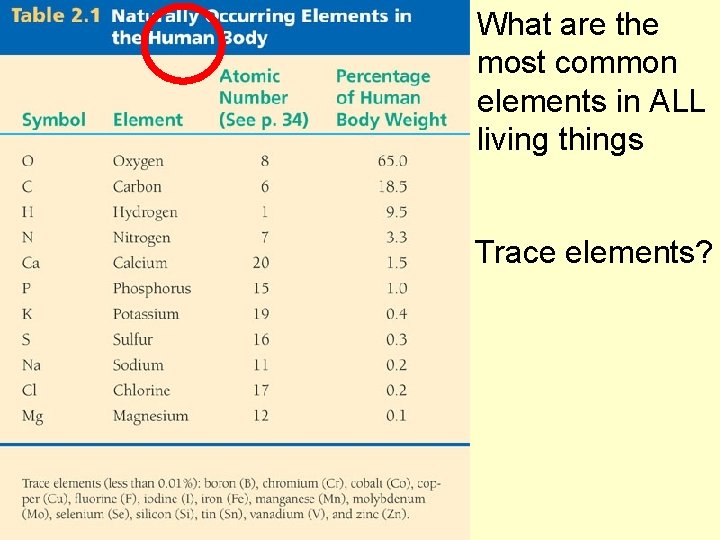
What are the most common elements in ALL living things Trace elements?

Symptom of an iodine deficiency Iron deficiency? Fluorine deficiency?
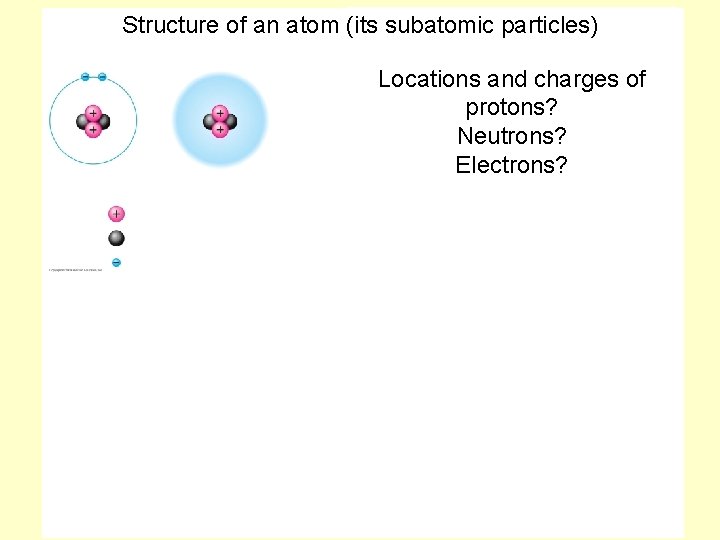
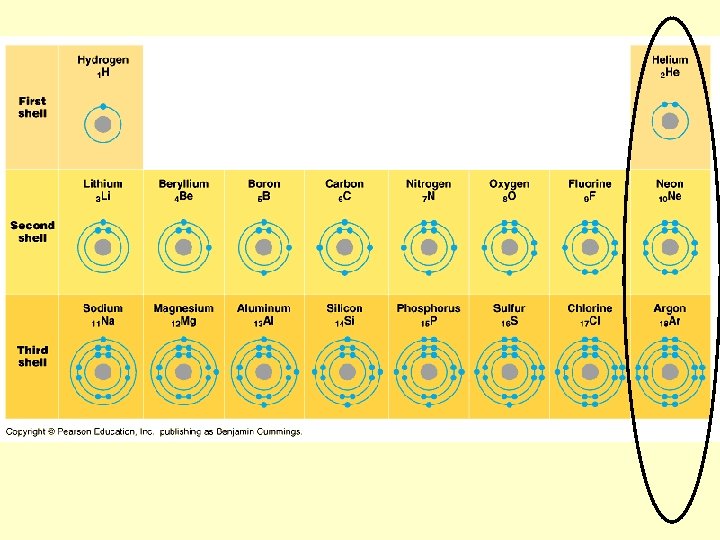
Structure of an atom (its subatomic particles) Locations and charges of protons? Neutrons? Electrons?
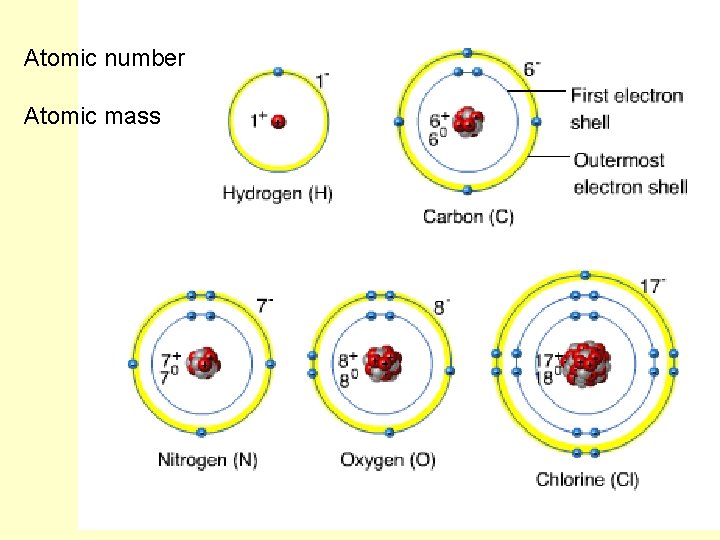
Atomic number Atomic mass
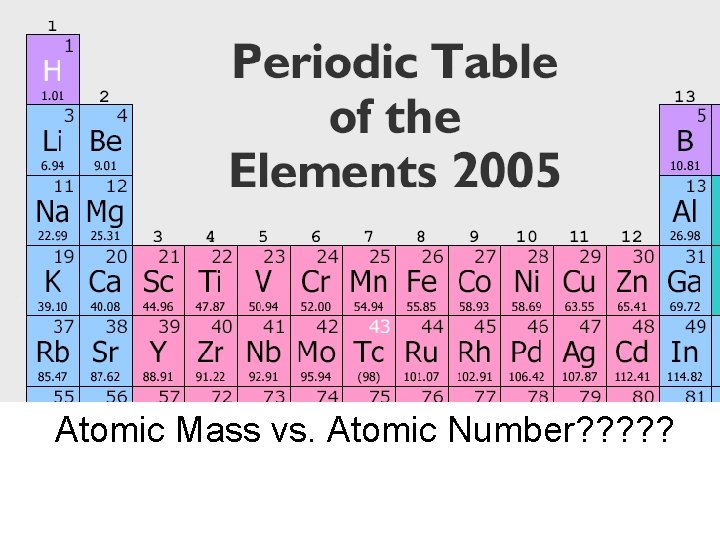
Atomic Mass vs. Atomic Number? ? ?

What is an isotope?
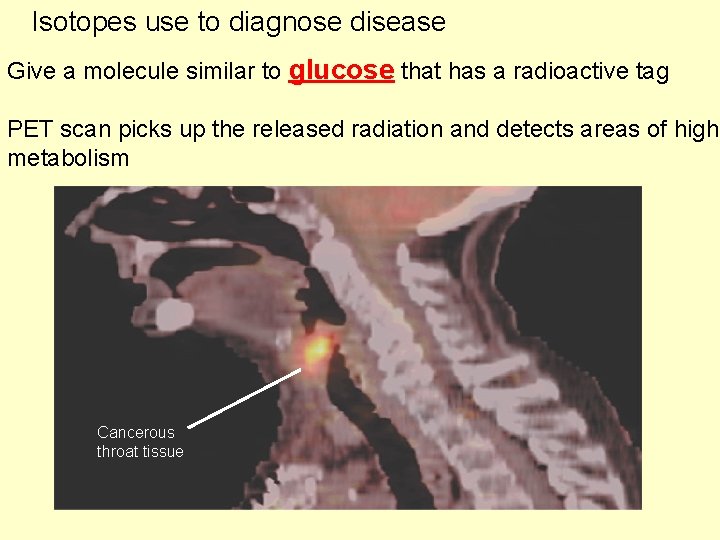
Isotopes use to diagnose disease Give a molecule similar to glucose that has a radioactive tag PET scan picks up the released radiation and detects areas of high metabolism Cancerous throat tissue

Radioactive iodine High doses can treat thyroid cancer Small doses used to test thyroid function.
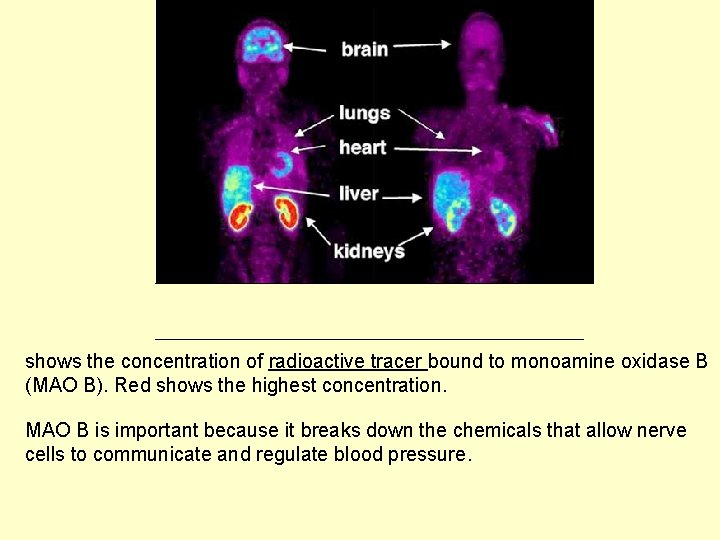
shows the concentration of radioactive tracer bound to monoamine oxidase B (MAO B). Red shows the highest concentration. MAO B is important because it breaks down the chemicals that allow nerve cells to communicate and regulate blood pressure.

What causes an atom to react with other atom? Or… would cause it to be nonreactive (stable)?
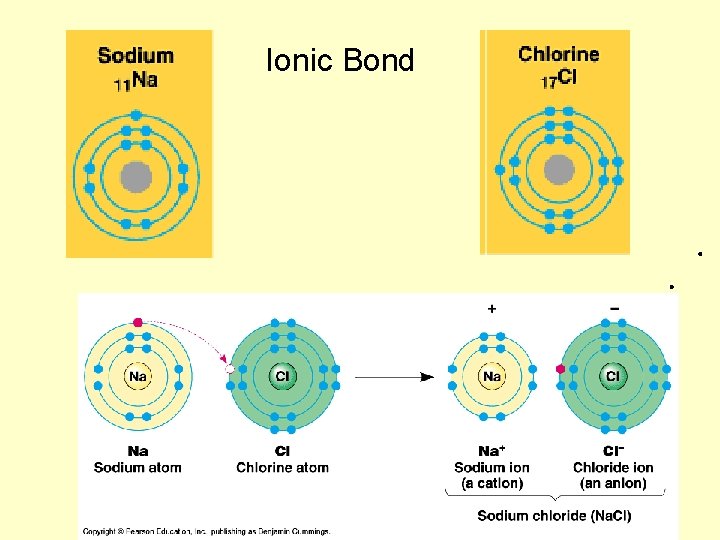
Ionic Bond


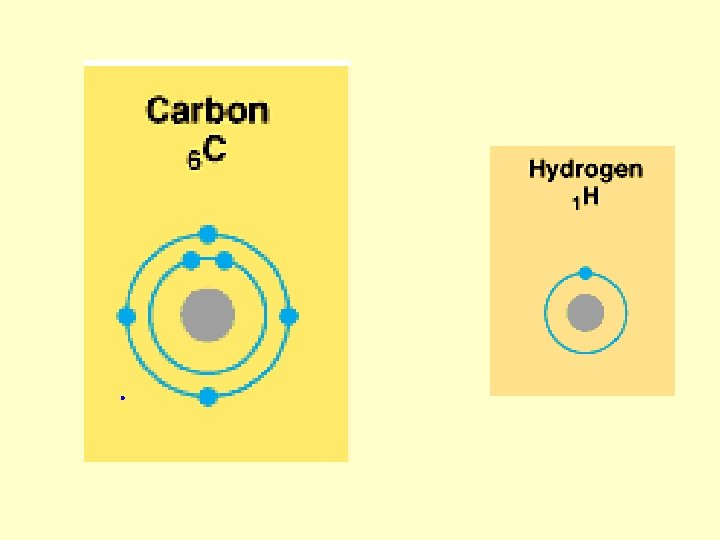
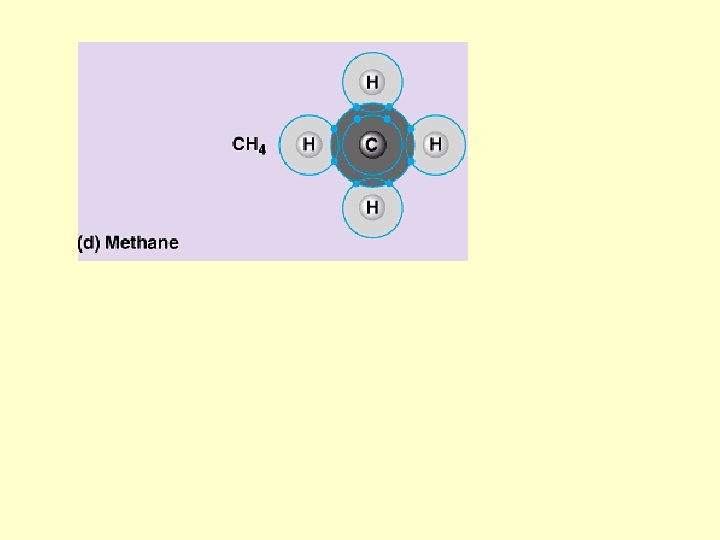


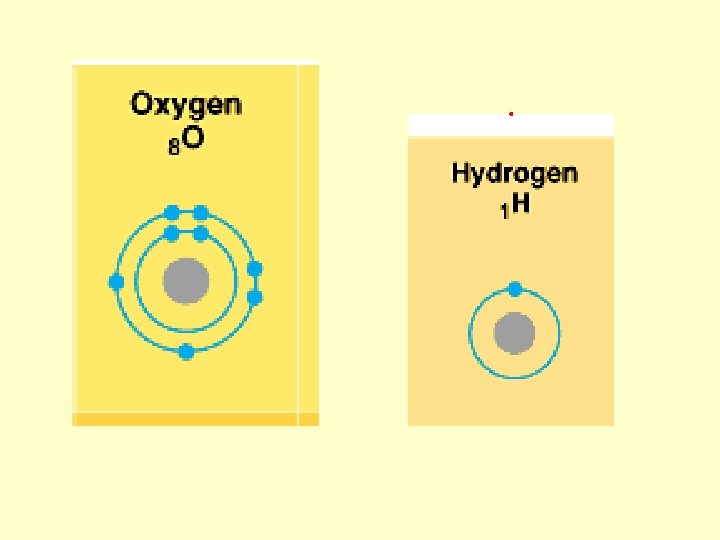
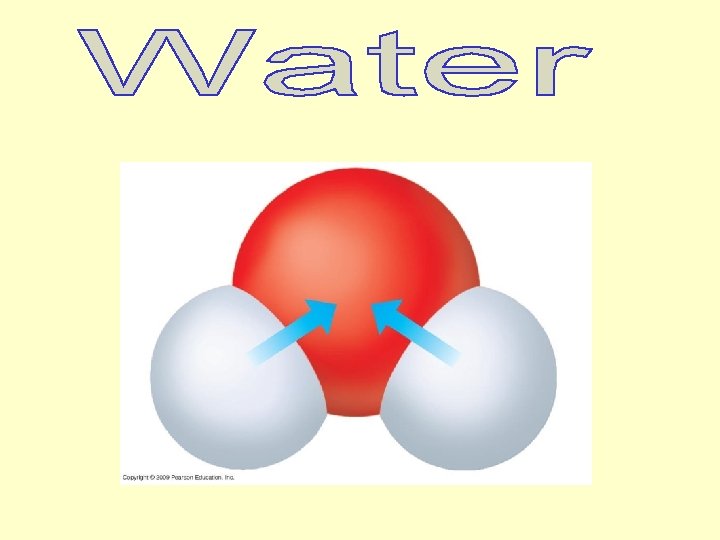
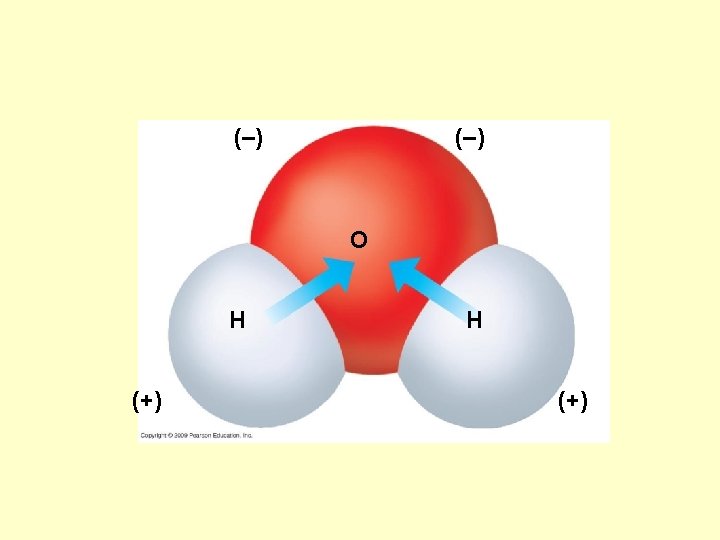
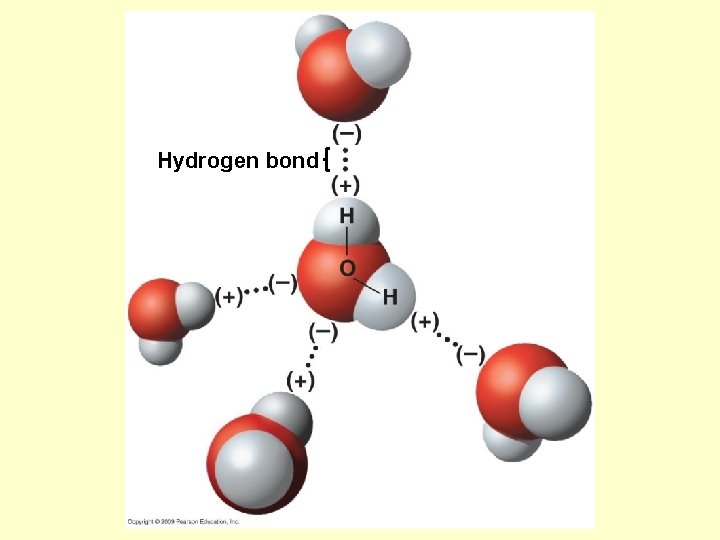
Hydrogen bond
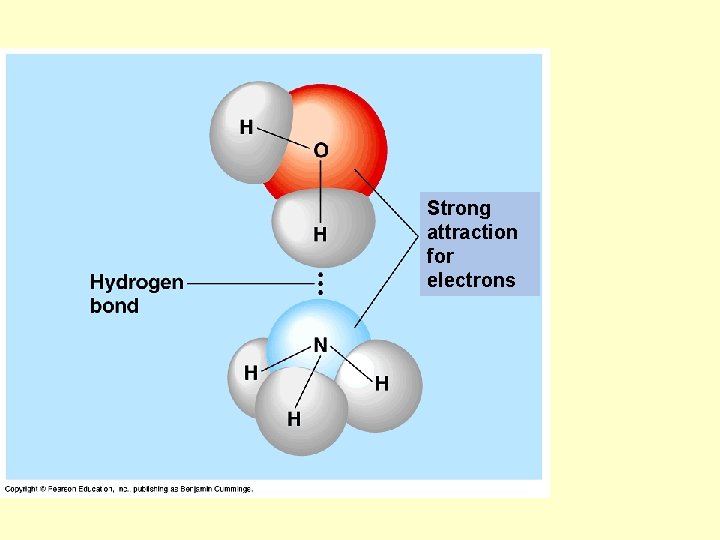
Strong attraction for electrons

Cohesion of water → water sticking to itself • Does this because of hydrogen bonding • Results in water having surface tension – “film” on top of water


Transpiration • Uses cohesion and adhesion to move water from the roots to top of a tree

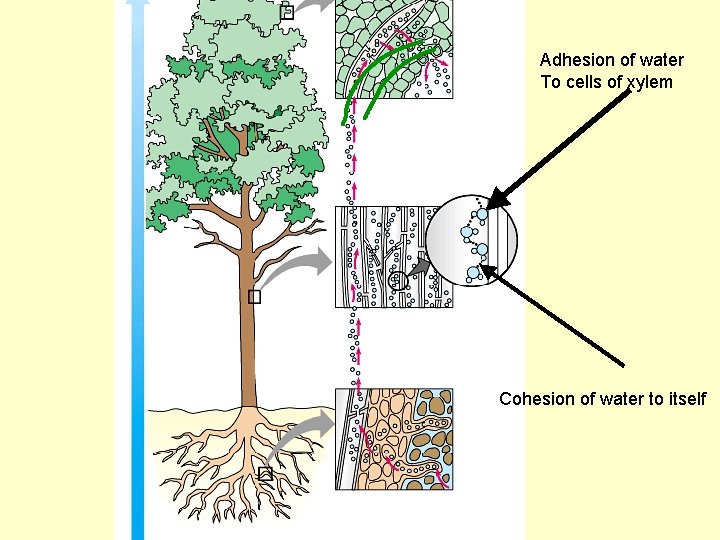
Adhesion of water To cells of xylem Cohesion of water to itself

Adhesion → water sticking to something else Meniscus

Temperature moderation Water resists changes in temperature Water can absorb and release a lot of thermal energy with a small change in temperature ex: lake temperatures

moderate temperatures at coasts Feb: 44. 3° F July: 65. 3 ° F Feb: 15. 7° F July: 70. 4 ° F

ex: evaporative cooling -when water evaporates from our skin, it takes a lot of thermal energy with it

Density Water is densest at 4° C Provides insulation and allows wildlife to survive under a layer of ice Ice is less dense than liquid water Ice floats
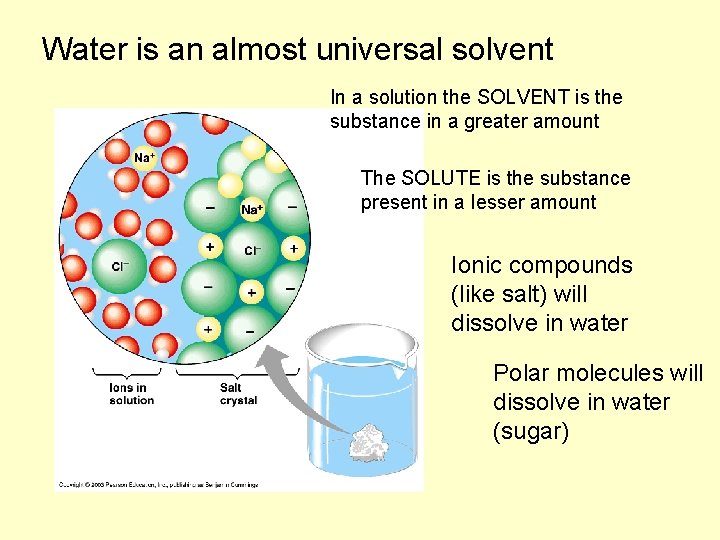
Water is an almost universal solvent In a solution the SOLVENT is the substance in a greater amount The SOLUTE is the substance present in a lesser amount Ionic compounds (like salt) will dissolve in water Polar molecules will dissolve in water (sugar)
Glucose
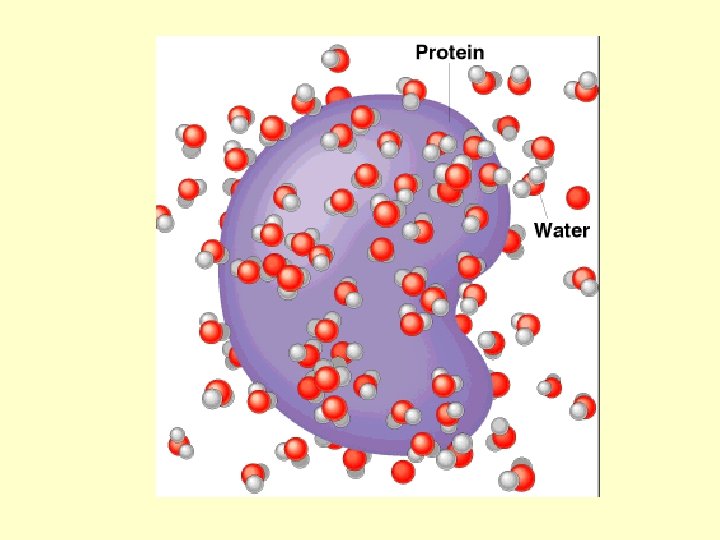
p. H • A few water molecules can break apart into ions – hydrogen ions (H+) – hydroxide ions (OH–) Copyright © 2009 Pearson Education, Inc.
p. H • Acids vs. bases – An acid is anything that INCREASES the relative concentration of H+ – A base is anything that DECREASES the relative concentration of H+ • Either by adding OH- or removing H+ Copyright © 2009 Pearson Education, Inc.
• A p. H scale (p. H = potential of hydrogen) is used to describe whether a solution is acidic or basic – p. H ranges from 0 (most acidic) to 14 (most basic) – A solution that is neither acidic or basic is neutral (p. H = 7) Copyright © 2009 Pearson Education, Inc.
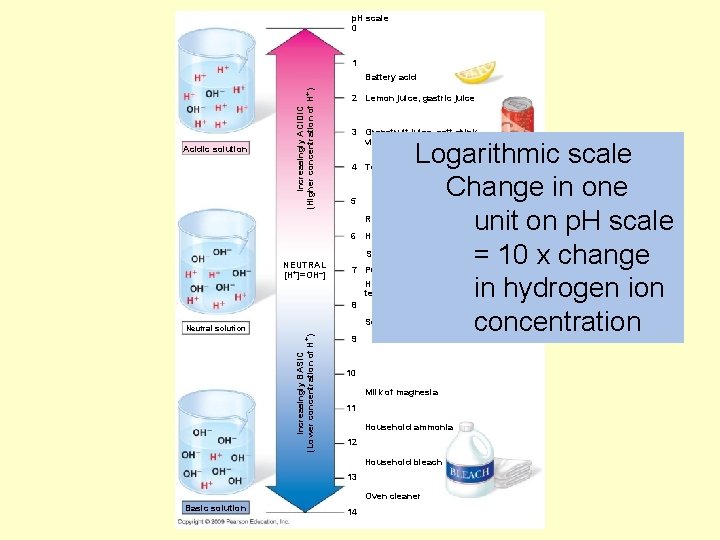
p. H scale 0 1 Acidic solution Increasingly ACIDIC (Higher concentration of H+) Battery acid 2 Lemon juice, gastric juice 3 Grapefruit juice, soft drink, vinegar, beer Logarithmic scale Change in one unit on p. H scale = 10 x change in hydrogen ion concentration 4 Tomato juice 5 Rain water 6 Human urine Saliva NEUTRAL [H+]=OH–] 7 Pure water Human blood, tears 8 Seawater Increasingly BASIC (Lower concentration of H+) Neutral solution 9 10 Milk of magnesia 11 Household ammonia 12 Household bleach 13 Oven cleaner Basic solution 14

Solution A has a p. H = 4 Solution B has a p. H = 2 How much more acidic is Solution B? Solution C has a p. H = 9 Solution D has a p. H = 12 How much more H+ ions does solution C have compared to solution D?
Why is p. H important? • Different areas of the body have different p. H values – Ex: stomach blood • If the p. H varies from this value, proteins lose their shape and don’t work
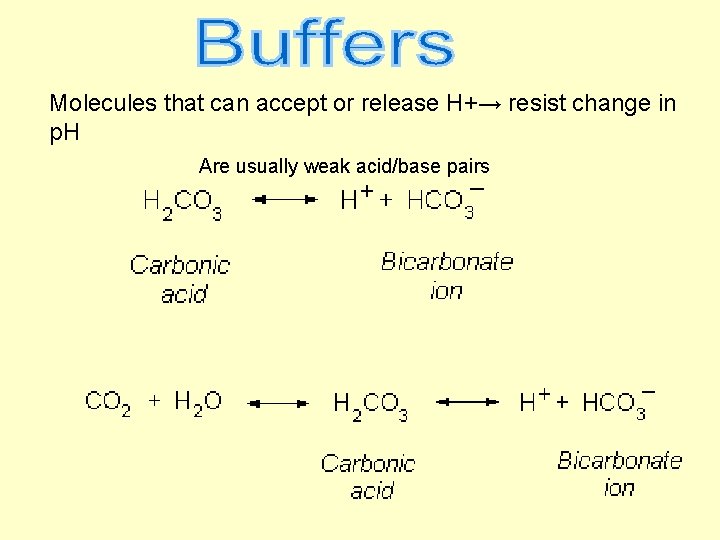
Molecules that can accept or release H+→ resist change in p. H Are usually weak acid/base pairs





- Slides: 53