Basic Chemistry Biochemistry Types of Compounds n Two

Basic Chemistry Biochemistry

Types of Compounds n Two types of compounds important to life: n Organic Compounds n Inorganic Compounds

Biochemistry: Essentials for Life • Organic compounds • Contain carbon • Most are covalently bonded • Example: C 6 H 12 O 6 (glucose) • Inorganic compounds • Lack carbon • Tend to be simpler compounds • Example: H 2 O (water) Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 21

Important Inorganic Compounds n Water n Salts n Acids/Bases

Important Inorganic Compounds • Water • Most abundant inorganic compounds • Vital properties • High heat capacity • Polarity/solvent properties • Chemical reactivity • Cushioning Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 22

Important Inorganic Compounds • Salts • Easily dissolve in the presence of water • Vital to many body functions • Include electrolytes which conduct electrical currents Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 23

Important Inorganic Compounds • Acids • Can release detectable hydrogen ions • Bases • Proton acceptors • Neutralization reaction • Acids and bases react to form water and a salt Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 24
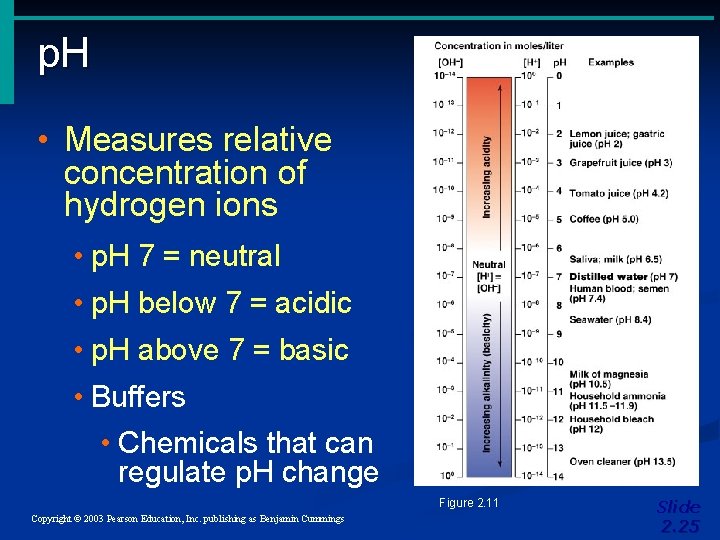
p. H • Measures relative concentration of hydrogen ions • p. H 7 = neutral • p. H below 7 = acidic • p. H above 7 = basic • Buffers • Chemicals that can regulate p. H change Figure 2. 11 Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 25

Challenge Problem 1. 2. 3. 4. Why is water so important to living things? Give one example of what salts do for living things. Based on what you know about inorganic compounds, why do people drink sports drinks during exercise? Give an example of a monosaccharide(simple sugar)

Important Organic Compounds n Carbohydrates n Lipids n Proteins n Nucleic n ATP Acids

Important Organic Compounds • Carbohydrates • Contain carbon, hydrogen, and oxygen • Include sugars and starches • Classified according to size • Monosaccharides – simple sugars • Disaccharides – two simple sugars joined by dehydration synthesis • Polysaccharides – long branching chains of linked simple sugars Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 26

Challenge Problem For two monosaccharides to bond together to form a disaccharide which reaction must occur? The building blocks of polysaccharides are _____. The following are organic or inorganic 1. 2. 3. - 4. HCl C 6 H 12 O 6 Na. Cl CH 4 Which of the following is a
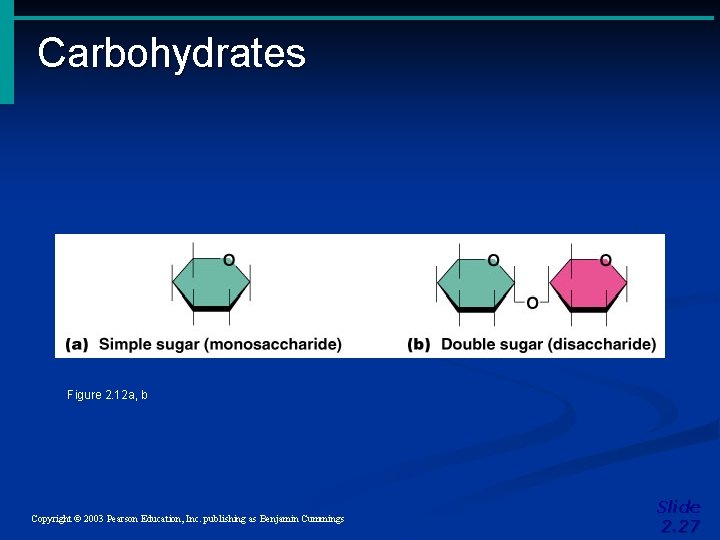
Carbohydrates Figure 2. 12 a, b Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 27
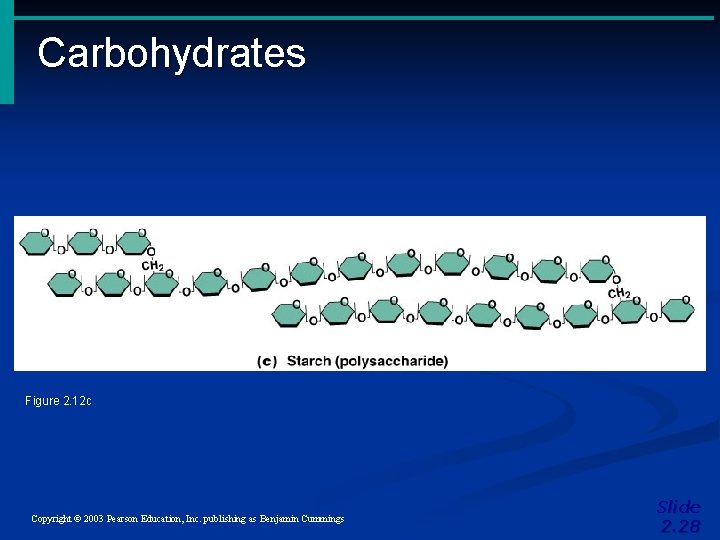
Carbohydrates Figure 2. 12 c Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 28

Important Organic Compounds • Lipids • Contain carbon, hydrogen, and oxygen • Carbon and hydrogen outnumber oxygen • Insoluble in water • Fats, Oils, and Waxes Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 29

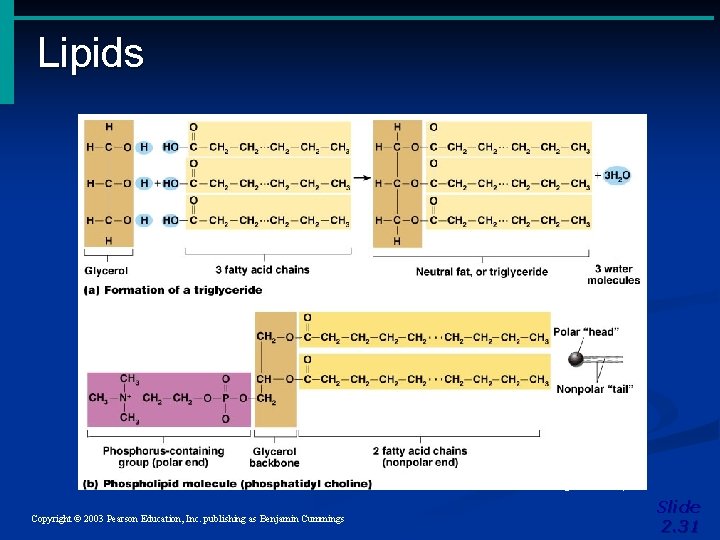
Lipids Figure 2. 14 a, b Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 31
Important Organic Compounds • Proteins • Made of amino acids • Contain carbon, oxygen, hydrogen, nitrogen, and sometimes sulfur Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 33 a

Important Organic Compounds PROTEINS • Account for over half of the body’s organic matter • Provides for construction materials for body tissues • Plays a vital role in cell function • Act as enzymes, hormones, and antibodies Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 33 b
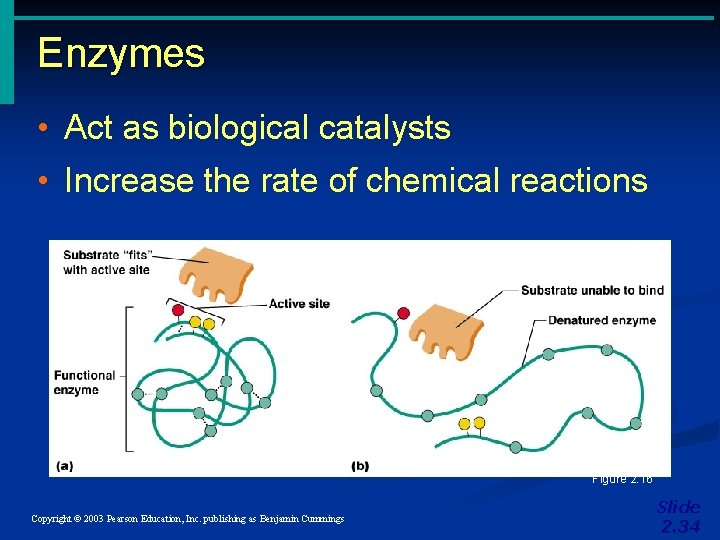
Enzymes • Act as biological catalysts • Increase the rate of chemical reactions Figure 2. 16 Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 34
Important Organic Compounds • Nucleic Acids • Provide blueprint of life • Nucleotide bases • A = Adenine • G = Guanine • C = Cytosine • T = Thymine • U = Uracil • Make DNA and RNA Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 35
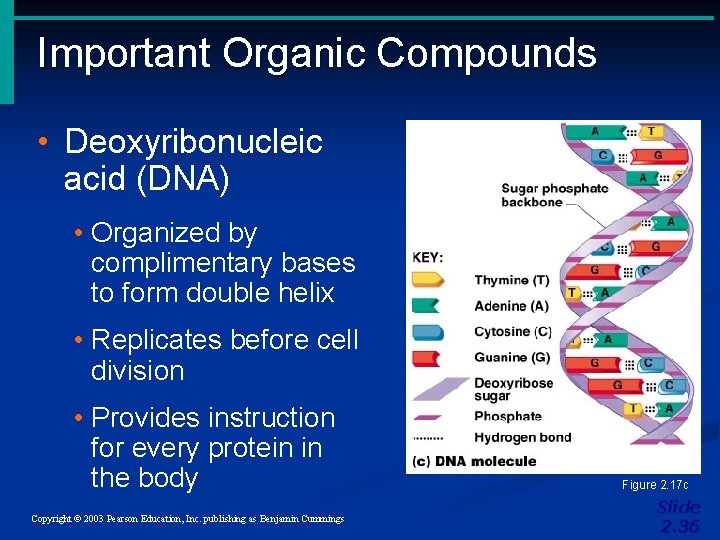
Important Organic Compounds • Deoxyribonucleic acid (DNA) • Organized by complimentary bases to form double helix • Replicates before cell division • Provides instruction for every protein in the body Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Figure 2. 17 c Slide 2. 36
Important Organic Compounds • Adenosine triphosphate (ATP) • Chemical energy used by all cells • Energy is released by breaking high energy phosphate bond • ATP is replenished by oxidation of food fuels Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 37
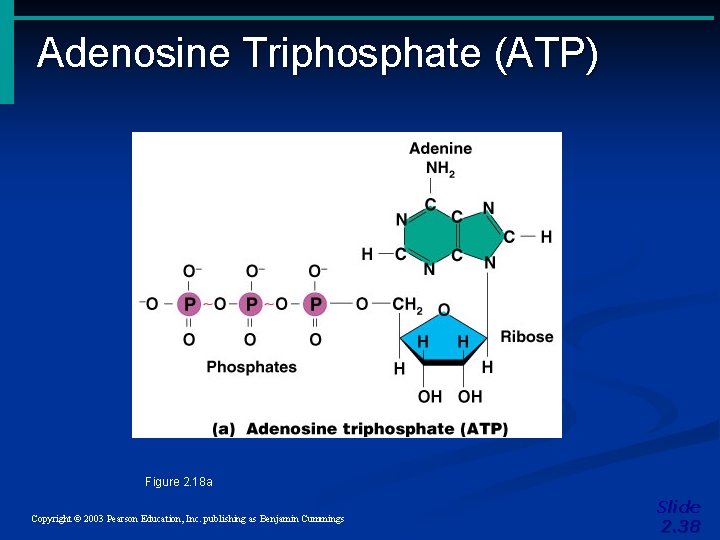
Adenosine Triphosphate (ATP) Figure 2. 18 a Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 38
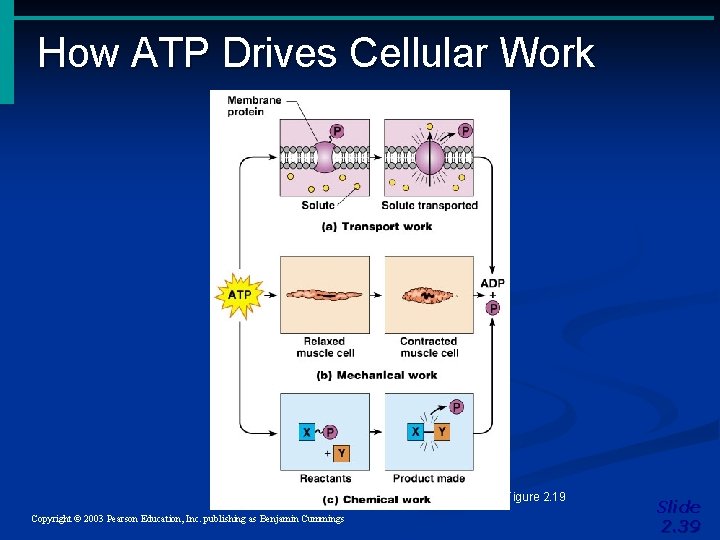
How ATP Drives Cellular Work Figure 2. 19 Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings Slide 2. 39

Quick Quiz 2 of 2 1. 2. 3. 4. Enzymes are made of what kind of important organic molecule? Why do we need carbohydrates? Give two examples of sources of lipids for humans Why is ATP such an important molecule?
- Slides: 26