Basic Atomic Structure What does the atom look
Basic Atomic Structure What does the atom look like?

Democritus: theorized a solid, indestructible object His atom looked something like this: It wasn’t until J. J. Thompson came around, that theory of atomic structure changed. Why? The discovery of protons & electrons
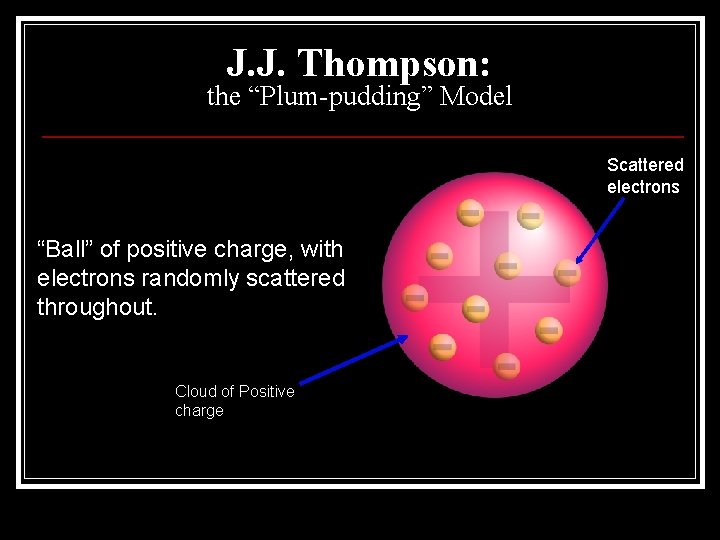
J. J. Thompson: the “Plum-pudding” Model Scattered electrons “Ball” of positive charge, with electrons randomly scattered throughout. Cloud of Positive charge
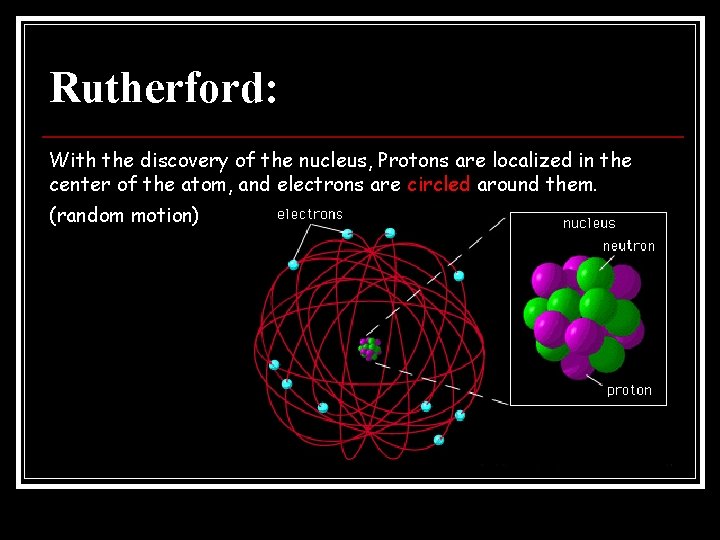
Rutherford: With the discovery of the nucleus, Protons are localized in the center of the atom, and electrons are circled around them. (random motion)
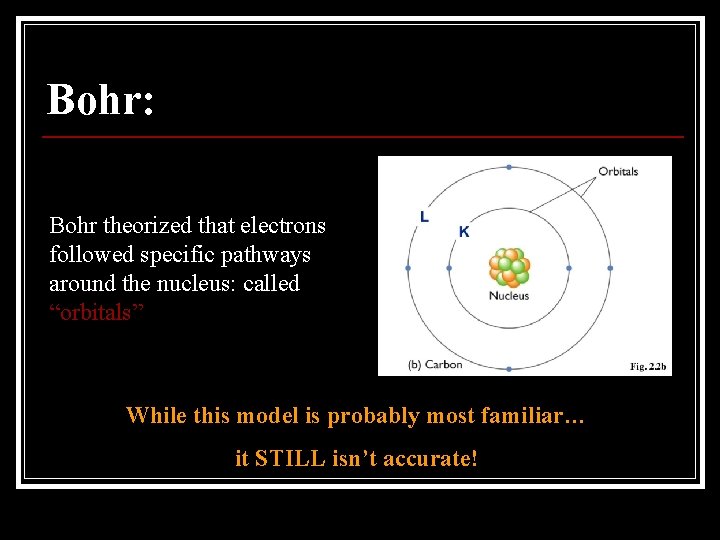
Bohr: Bohr theorized that electrons followed specific pathways around the nucleus: called “orbitals” While this model is probably most familiar… it STILL isn’t accurate!
We will revisit WHY Bohr’s model is inaccurate, but for now… 3 subatomic particles: Protons & neutrons are about Proton (p+ - ‘+’ charge) Electron (e- - ‘-’ charge) Neutron (no - no charge) the same size & mass. Each has a mass of 1 a. m. u. (atomic mass unit) Electrons are 1/1780 the size of a proton. Effectively 0 a. m. u.
Protons & neutrons are found in the center of the atom: the nucleus • The number of protons tells you what type of atom you have: the atomic number • The protons determine what type of atom you have!!! • The mass of an atom is calculated by adding the number of protons + neutrons: The mass number How are atoms of one element different than the atoms of a different element?

Atoms are the smallest form of an element to retain those properties of that element: This means the smallest piece of gold I can have, that will act like gold, is 1 atom of gold. The different kinds of elements are found on the Periodic Table of the Elements are organized on the Periodic Table by Atomic Number. (# of p+’s)
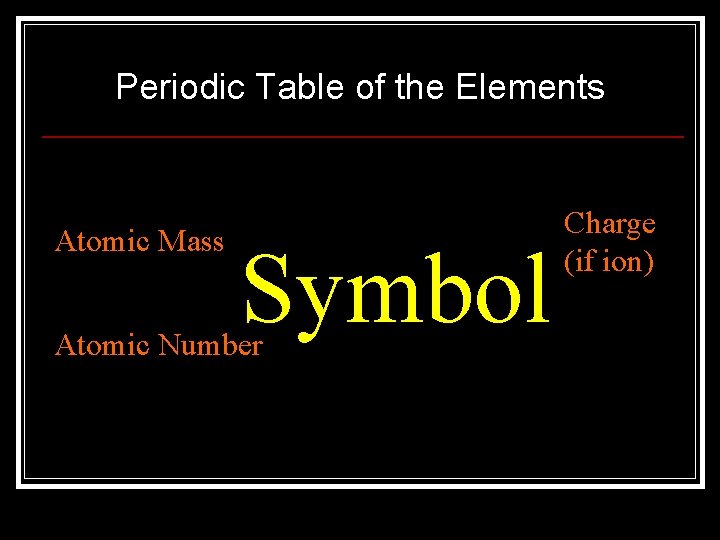
Periodic Table of the Elements Atomic Mass Symbol Atomic Number Charge (if ion)
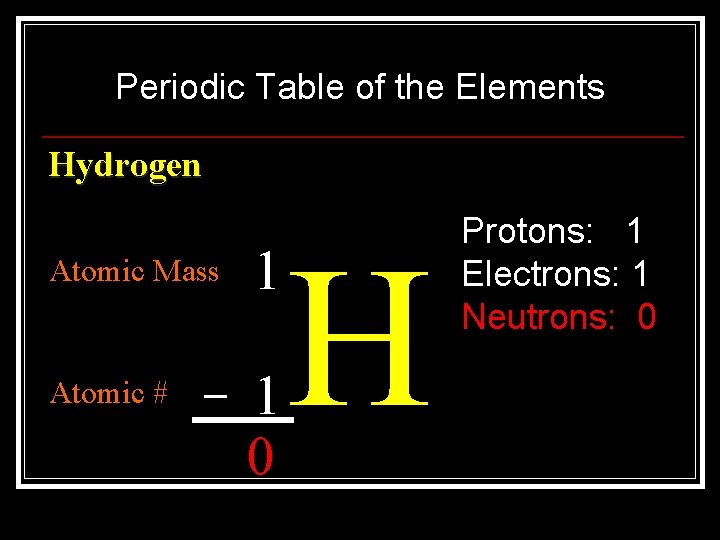
Periodic Table of the Elements Hydrogen Atomic Mass 1 _ 1 0 Atomic # H Protons: 1 Electrons: 1 Neutrons: 0
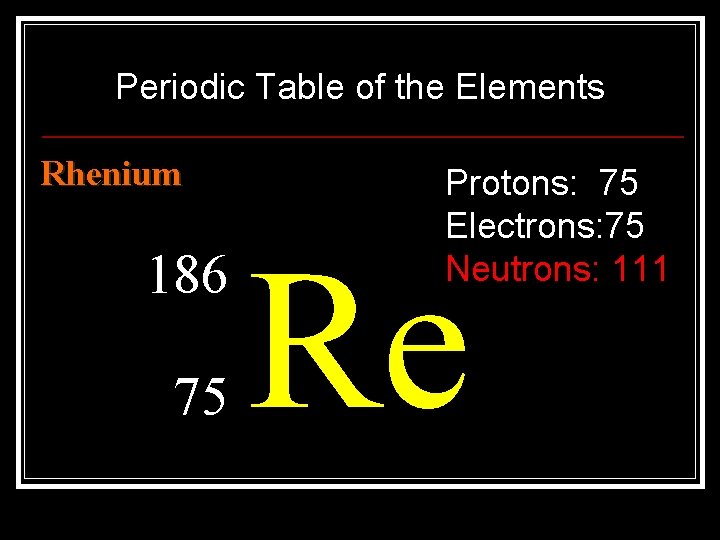
Periodic Table of the Elements Rhenium 186 75 Protons: 75 Electrons: 75 Neutrons: 111 Re
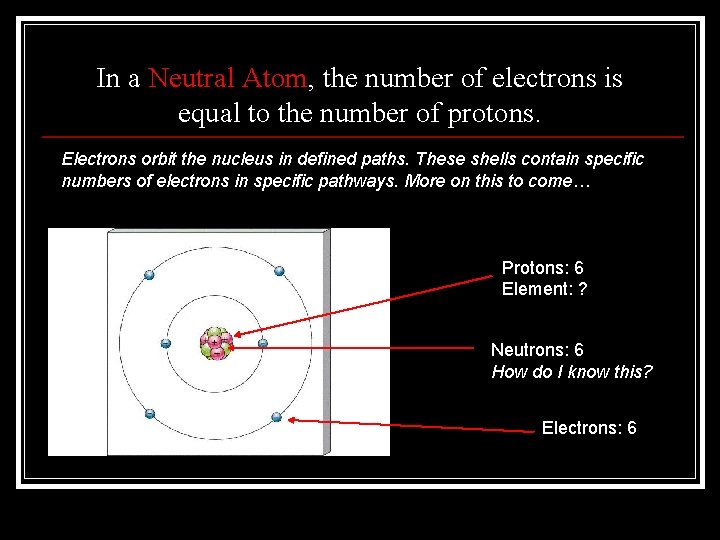
In a Neutral Atom, the number of electrons is equal to the number of protons. Electrons orbit the nucleus in defined paths. These shells contain specific numbers of electrons in specific pathways. More on this to come… Protons: 6 Element: ? Neutrons: 6 How do I know this? Electrons: 6
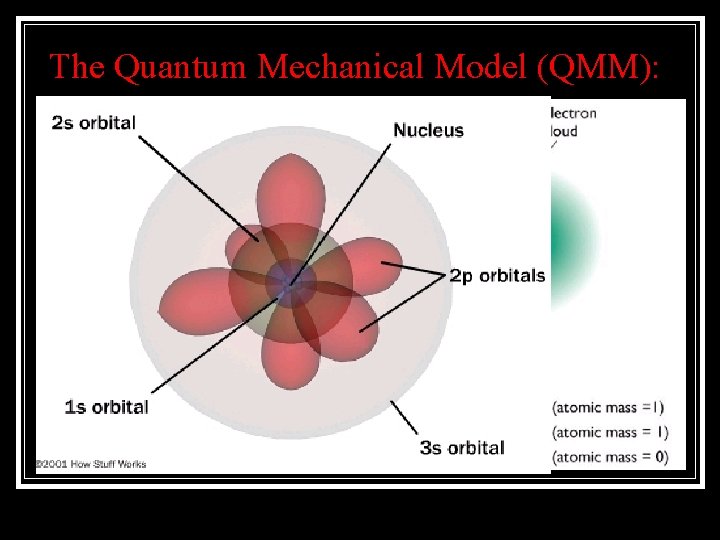
The Quantum Mechanical Model (QMM): This model states that electrons still follow specific pathways, these orbitals, have specific 3 D shapes: It also says that because you can never know the speed of an electron & its position at the same time, you could only guess where an electron was likely to be 90% of the time:
- Slides: 14