Basic Atomic Chemistry Early Chemistry Early Chemists only

Basic Atomic Chemistry
Early Chemistry Ø Early Chemists only believed in 1 element Ø Later Chemists believed in 4 elements: l l Earth Air Fire Water
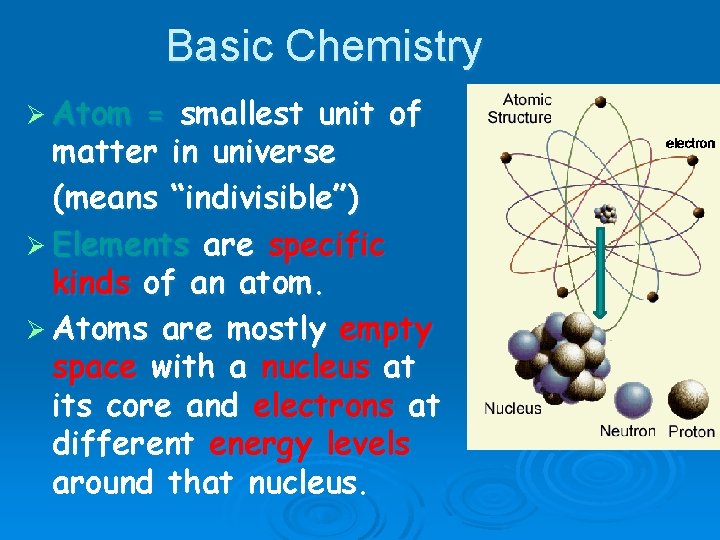
Basic Chemistry Ø Atom = smallest unit of matter in universe (means “indivisible”) Ø Elements are specific kinds of an atom. Ø Atoms are mostly empty space with a nucleus at its core and electrons at different energy levels around that nucleus.
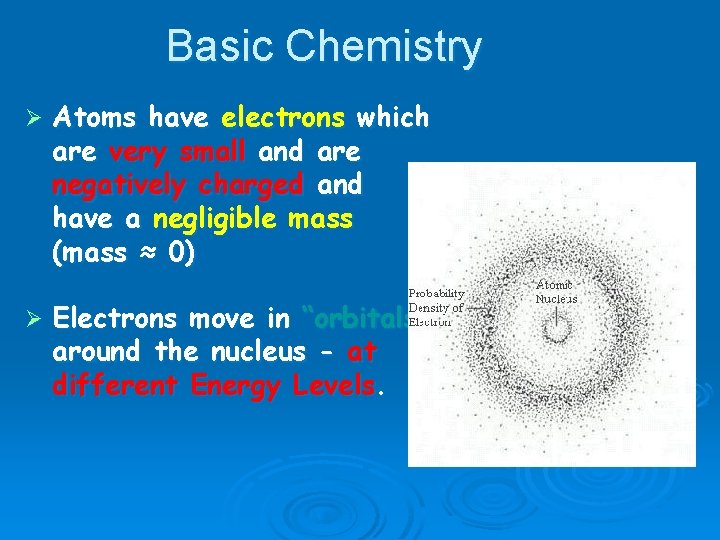
Basic Chemistry Ø Atoms have electrons which are very small and are negatively charged and have a negligible mass (mass ≈ 0) Ø Electrons move in “orbitals” around the nucleus - at different Energy Levels.
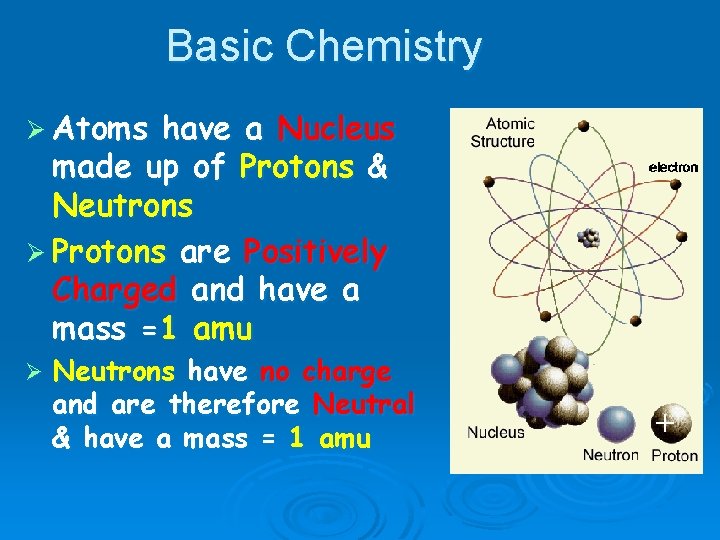
Basic Chemistry Ø Atoms have a Nucleus made up of Protons & Neutrons Ø Protons are Positively Charged and have a mass =1 amu Ø Neutrons have no charge and are therefore Neutral & have a mass = 1 amu +
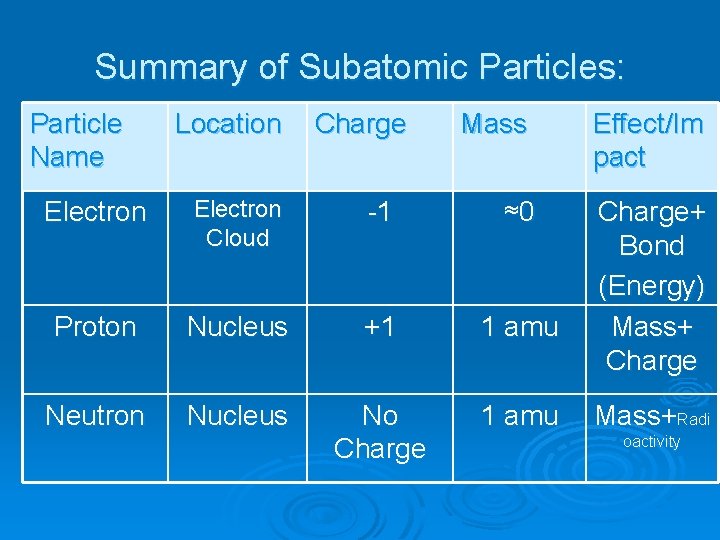
Summary of Subatomic Particles: Particle Name Location Charge Mass Effect/Im pact Charge+ Bond (Energy) Mass+ Charge Electron Cloud -1 ≈0 Proton Nucleus +1 1 amu Neutron Nucleus No Charge 1 amu Mass+Radi oactivity
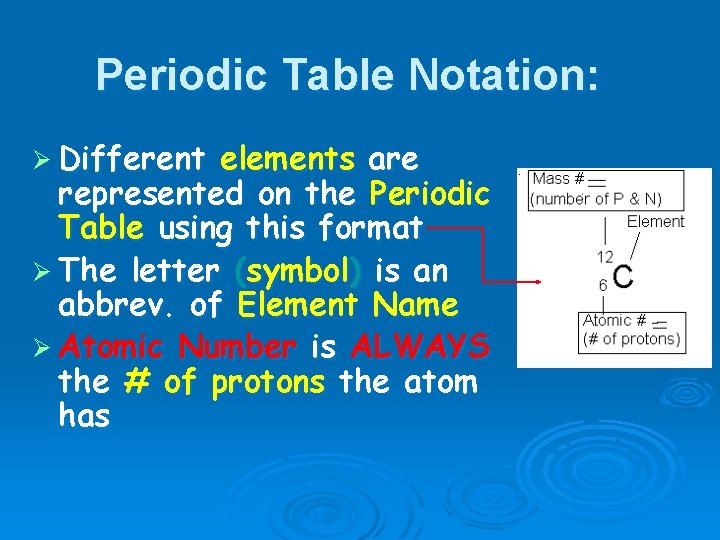
Periodic Table Notation: Ø Different elements are represented on the Periodic Table using this format Ø The letter (symbol) is an abbrev. of Element Name Ø Atomic Number is ALWAYS the # of protons the atom has

Atomic Number Determines the specific identity of an element. Ø Examples: l Carbon = 6 l Hydrogen = 1 l Oxygen = 8 l Nitrogen = 7 Ø
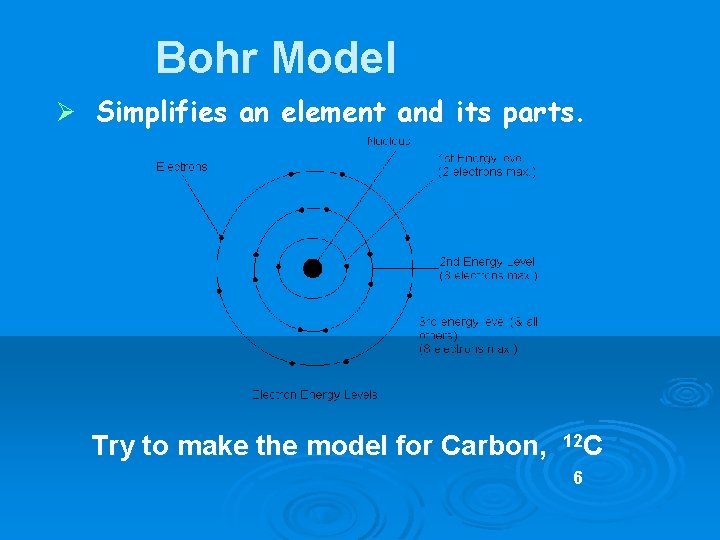
Bohr Model Ø Simplifies an element and its parts. Try to make the model for Carbon, 12 C 6

“Forms” of atoms Ø About 100 different elements in Per. Table … but about 2000 forms of those 100. 1. ION: same atom w/ a varying # of electrons. Neutral atoms have EQUAL numbers of protons (+) and electrons ( -) so overall charge = 0. + ions lose e’s & – ions gain e’s.
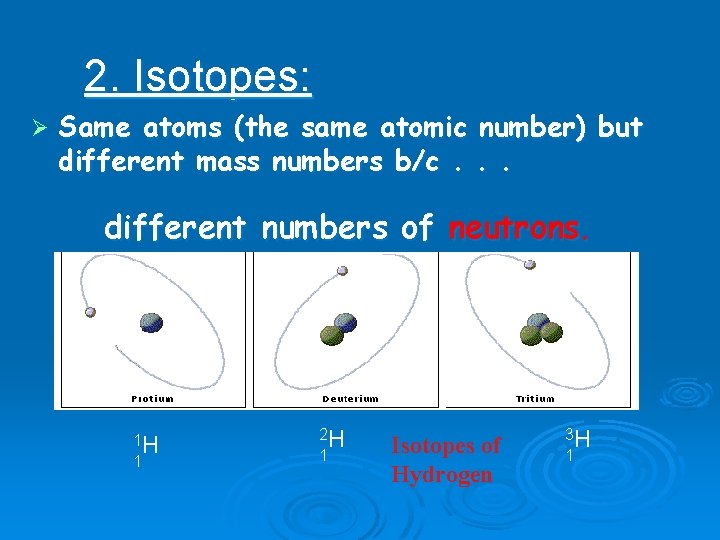
2. Isotopes: Ø Same atoms (the same atomic number) but different mass numbers b/c. . . different numbers of neutrons. 1 H 1 2 H 1 Isotopes of Hydrogen 3 H 1
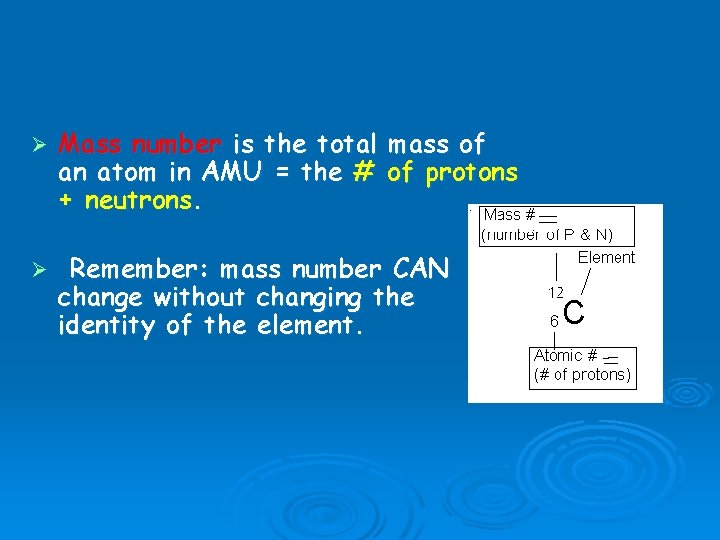
Ø Mass number is the total an atom in AMU = the # + neutrons. mass of of protons Ø Remember: mass number CAN change without changing the identity of the element.
Average Atomic Mass Ø The atomic mass on the Periodic Table is not a whole number. Ø When atomic mass is calculated scientists use a weighted average Calculated by adding the masses of each isotope × their relative abundances

Practice Calculation Ø On board

Ex: Hydrogen has an average atomic mass = 1. 0079. This means that most hydrogens have an mass of 1 but a few = 2 and even fewer = 3. Thus, the average is slightly above 1. Q: What if hydrogen’s average atomic mass = 1. 95? What can we infer? MARBLE LAB

Radioactive isotopes Ø = unstable nucleus which decays and gives off radiation energy. Ø How are isotopes used in biology? 1. As tracers—organisms use elements regardless of it isotopic form, so scientists can label and follow a chemical’s path.
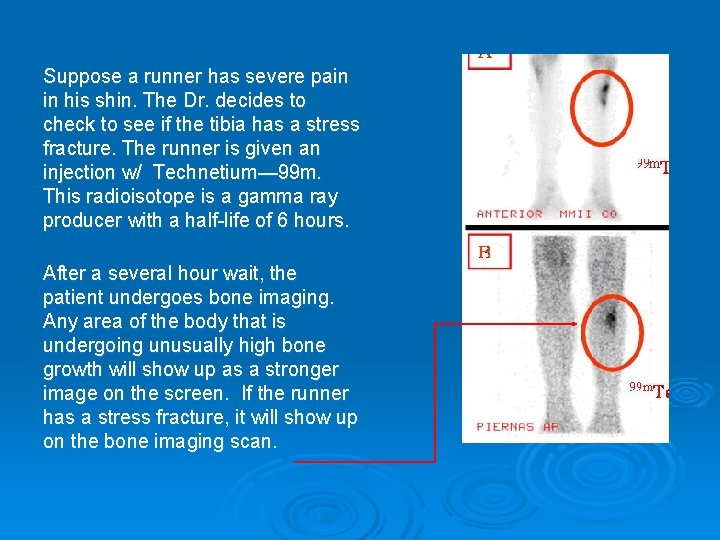
Suppose a runner has severe pain in his shin. The Dr. decides to check to see if the tibia has a stress fracture. The runner is given an injection w/ Technetium— 99 m. This radioisotope is a gamma ray producer with a half-life of 6 hours. After a several hour wait, the patient undergoes bone imaging. Any area of the body that is undergoing unusually high bone growth will show up as a stronger image on the screen. If the runner has a stress fracture, it will show up on the bone imaging scan.

2. Verifying Climate Change is in part human caused. #1 In the last 150 years we have increased the production of CO 2 via industrial waste and by deforestation (we can measure how much).
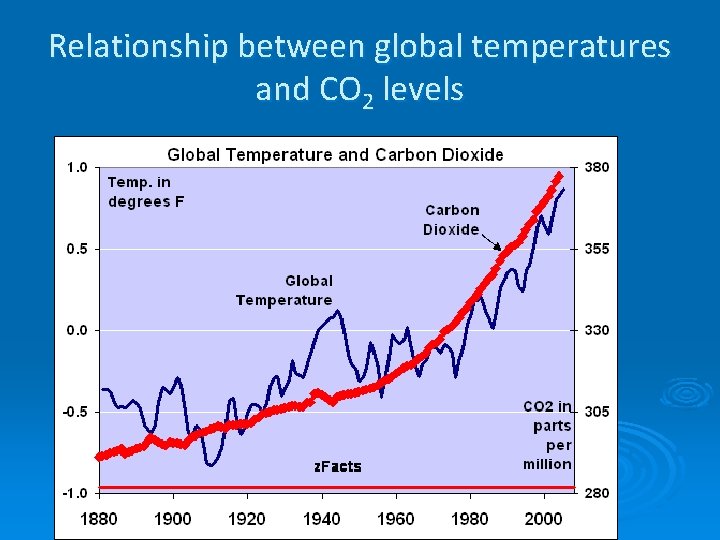
Relationship between global temperatures and CO 2 levels
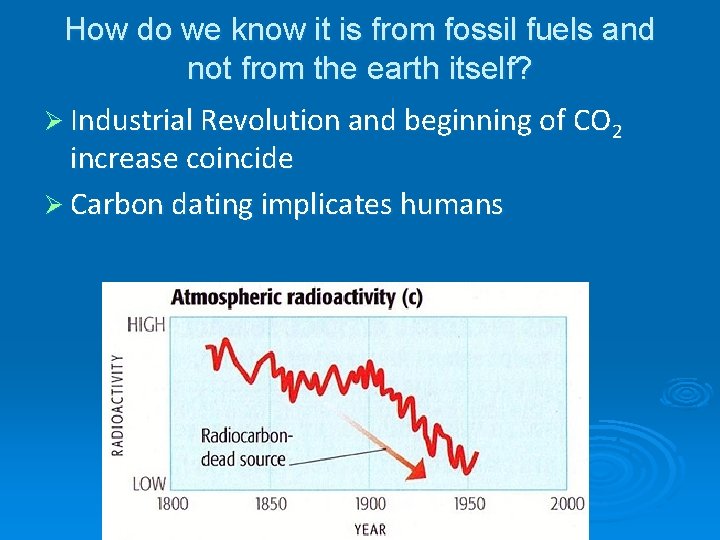
How do we know it is from fossil fuels and not from the earth itself? Ø Industrial Revolution and beginning of CO 2 increase coincide Ø Carbon dating implicates humans

Ø CO 2 from burning fossil fuels or forests has quite a different isotopic composition from CO 2 already in the atmosphere--because plants have a preference for the lighter (faster moving) isotopes (12 C vs. 13 C). Thus they have lower 13 C/12 C ratios than that of the atmosphere.

Ø Since fossil fuels are derived from ancient plants, plants and fossil fuels all have roughly the same 13 C/12 C ratio – about 2% lower than that of the atmosphere. As CO 2 from these materials is released and mixes with the atmosphere, the average 13 C/12 C ratio of the atmosphere has decreased (Real Climate: Climate Science from Climate Scientists)

3. Radiometric dating (p. 540 -1) Every unstable isotope decays at a constant rate called half-life = the time it takes a given amount of an isotope to decay to half of its original amount.

Example Ø Carbon dating. C-14 and C-12 are two different isotopes of carbon. The ratio of C -12 to C-14 in living tissue is relatively constant from one organism to the next. But what happens after the organism dies? Ø The amount of C-14 will go down compared to the amount of C-12. Why?

Practice Problems (in notes ok) Ø The ½ life of Carbon-14 ≈ 5730 years. How old would a fossil be if it contains 1 g of C-14 compared to a living sample of equal mass that has 8 g of C-14? Ø What amount (% or fraction) of C-14 would you be left after 4 half-lives? Ø HW: p. 569 #6, 7

Honors Ø Go to this web site: http: //www. physlink. com/Education/ask. Experts/ae 403. cfm <or use the internet to research> Answer these questions. 1. What does C-14 decay into? 2. If C-14 is decaying, why hasn’t it just all disappeared over time? 3. Why is C-14 not good to use when dating things less than a 10 years or more than 1 million years old?
- Slides: 26