Based on the Structure of Polymers There are
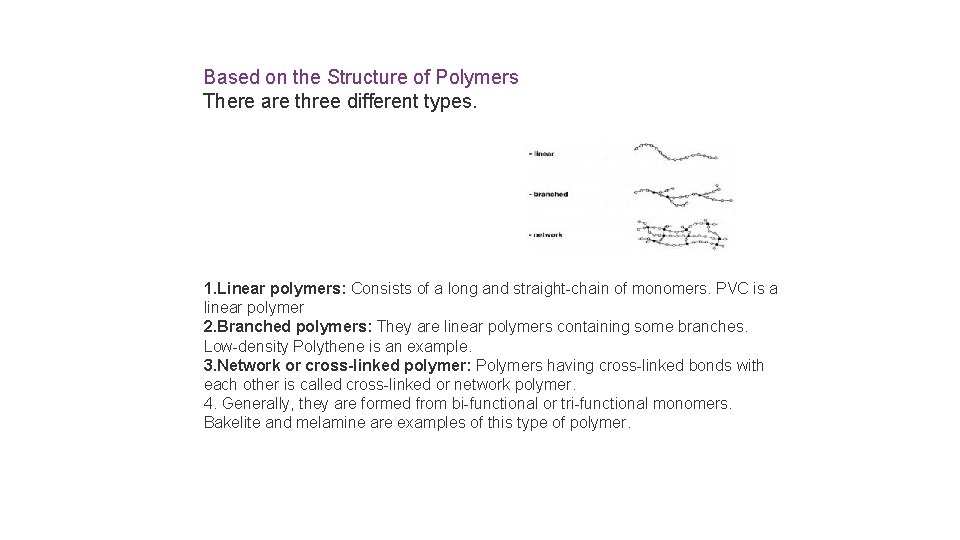
Based on the Structure of Polymers There are three different types. 1. Linear polymers: Consists of a long and straight-chain of monomers. PVC is a linear polymer 2. Branched polymers: They are linear polymers containing some branches. Low-density Polythene is an example. 3. Network or cross-linked polymer: Polymers having cross-linked bonds with each other is called cross-linked or network polymer. 4. Generally, they are formed from bi-functional or tri-functional monomers. Bakelite and melamine are examples of this type of polymer.
• Based on Mode of Polymerization • They are divided into two subcategories: • Addition Polymers: Polymers formed by the repeated addition of monomers by possessing the double or triple bonds. If the addition is of the same species they are called homopolymers and if the addition is of different monomers they are called copolymers. Examples are polythene and Buna-s respectively. • Condensation polymers: These polymers are formed by repeated condensation of tri or bifunctional monomeric units. In this reaction elimination of some small molecules like water and hydrogen chloride etc will take place. Terylene and Nylon 6, 6 are examples.
• Based on Forces Between Molecules • They are again classified into four subgroups. • Elastomers: Polymers that are rubber-like solids and having elastic properties. Here the polymer bonds are held together by weak intermolecular forces and that allows these polymers to stretch. The cross-links present in the polymer between the chains helps to retrace the original position after the removal of the applied force. Examples are Buna-s and Buna-s • Fibres: They are polymers having strong intermolecular forces like hydrogen bonding. Due to this strong force molecules are kept closer, that is they are closely packed. Because of this property, they are crystalline in nature. Polyamide and polyesters are examples. • Thermoplastic polymers: These are the liner or slightly changed to branched polymers that can be softened on continues heating and hardening on cooling. Their intermolecular force lying in between the fibres and elastomers. Polyvinyls, polystyrene etc are examples of thermoplastic polymers. • Thermosetting polymers: Polymers comes under the category of heavily branched or cross-linked, which can mould on heating and can’t regain the original shape. So these cannot be reused. Bakelite is an example.

• Plastic, polymeric material that has the capability of being molded or shaped, usually by the application of heat and pressure. This property of plasticity, often found in combination with other special properties such as low density, low electrical conductivity, transparency, and toughness, allows plastics to be made into a great variety of products. These include tough and lightweight beverage bottles made of polyethylene terephthalate (PET), flexible garden hoses made of polyvinyl chloride (PVC), insulating food containers made of foamed polystyrene, and shatterproof windows made of polymethyl methacrylate. • In this article a brief review of the essential properties of plastics is provided, followed by a more detailed description of their processing into useful products and subsequent recycling. For a fuller understanding of the materials from which plastics are made, see chemistry of industrial polymers.

• The Composition, Structure, And Properties Of Plastics • Many of the chemical names of the polymers employed as plastics have become familiar to consumers, although some are better known by their abbreviations or trade names. Thus, polyethylene terephthalate and polyvinyl chloride are commonly referred to as PET and PVC, while foamed polystyrene and polymethyl methacrylate are known by their trademarked names, Styrofoam and Plexiglas (or Perspex).

• Industrial fabricators of plastic products tend to think of plastics as either “commodity” resins or “specialty” resins. (The term resin dates from the early years of the plastics industry; it originally referred to naturally occurring amorphous solids such as shellac and rosin. ) Commodity resins are plastics that are produced at high volume and low cost for the most common disposable items and durable goods. They are represented chiefly by polyethylene, polypropylene, polyvinyl chloride, and polystyrene. Specialty resins are plastics whose properties are tailored to specific applications and that are produced at low volume and higher cost. Among this group are the so-called engineering plastics, or engineering resins, which are plastics that can compete with die-cast metals in plumbing, hardware, and automotive applications. Important engineering plastics, less familiar to consumers than the commodity plastics listed above, are polyacetal, polyamide (particularly those known by the trade name nylon), polytetrafluoroethylene (trademark Teflon), polycarbonate, polyphenylene sulfide, epoxy, and polyetherketone. Another member of the specialty resins is thermoplastic elastomers, polymers that have the elastic properties of rubber yet can be molded repeatedly upon heating. Thermoplastic elastomers are described in the article elastomer.

• Plastics also can be divided into two distinct categories on the basis of their chemical composition. One category is plastics that are made up of polymers having only aliphatic (linear) carbon atoms in their backbone chains. All the commodity plastics listed above fall into this category. The structure of polypropylene can serve as an example; here attached to every other carbon atom is a pendant methyl group (CH 3):
- Slides: 7