Balancing Redox Reactions using the Reaction Method Half

Balancing Redox Reactions using the ½ Reaction Method

Half Reaction • Half reaction – one of the two parts of a redox reaction • One half will be oxidation • One half will be reduction

Step to Balancing Half Reaction Method 1. Separate the reactions into 2 half reactions (one for oxidation & one for reduction) 2. Balance each ½ reaction in the following order: a) b) c) d) Balance elements other than H & O Balance O by adding H 2 O Balance H by adding H+ Balance the charge to make each side equal

Step to Balancing Half Reaction Method 1. Multiply each ½ reaction by a number to make the number of electrons gained = the number of electrons lost 2. Add the ½ reactions 3. Simplify 4. Check your work 5. Tip – Break things into ions or binary compounds
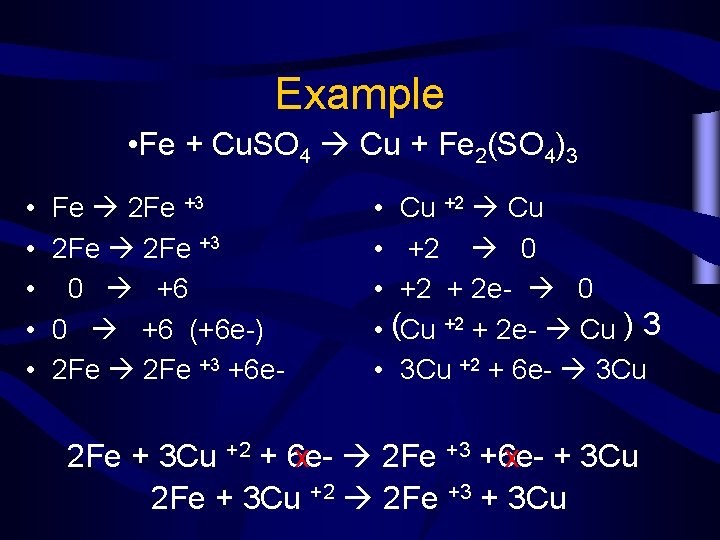
Example • Fe + Cu. SO 4 Cu + Fe 2(SO 4)3 • • • Fe 2 Fe +3 0 +6 (+6 e-) 2 Fe +3 +6 e- • Cu +2 Cu • +2 0 • +2 + 2 e- 0 • (Cu +2 + 2 e- Cu ) 3 • 3 Cu +2 + 6 e- 3 Cu 2 Fe + 3 Cu +2 + 6 ex 2 Fe +3 +6 ex + 3 Cu 2 Fe + 3 Cu +2 2 Fe +3 + 3 Cu
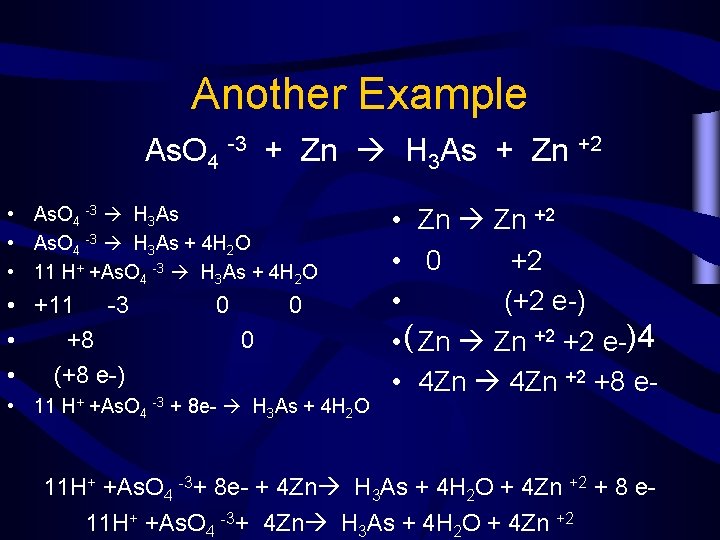
Another Example As. O 4 -3 + Zn H 3 As + Zn +2 • As. O 4 -3 H 3 As + 4 H 2 O • 11 H+ +As. O 4 -3 H 3 As + 4 H 2 O • +11 -3 • +8 • (+8 e-) 0 0 0 • 11 H+ +As. O 4 -3 + 8 e- H 3 As + 4 H 2 O • Zn +2 • 0 +2 • (+2 e-) • ( Zn +2 +2 e-)4 • 4 Zn +2 +8 e- 11 H+ +As. O 4 -3+ 8 e- + 4 Zn H 3 As + 4 H 2 O + 4 Zn +2 + 8 e 11 H+ +As. O 4 -3+ 4 Zn H 3 As + 4 H 2 O + 4 Zn +2
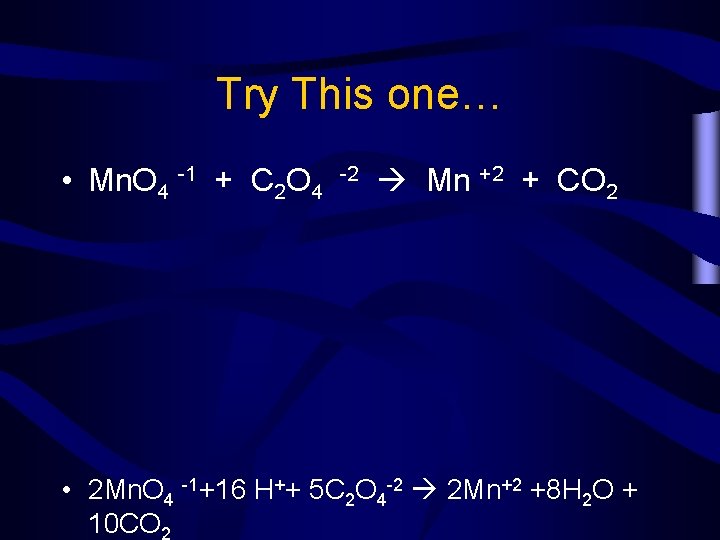
Try This one… • Mn. O 4 -1 + C 2 O 4 -2 Mn +2 + CO 2 • 2 Mn. O 4 -1+16 H++ 5 C 2 O 4 -2 2 Mn+2 +8 H 2 O + 10 CO 2
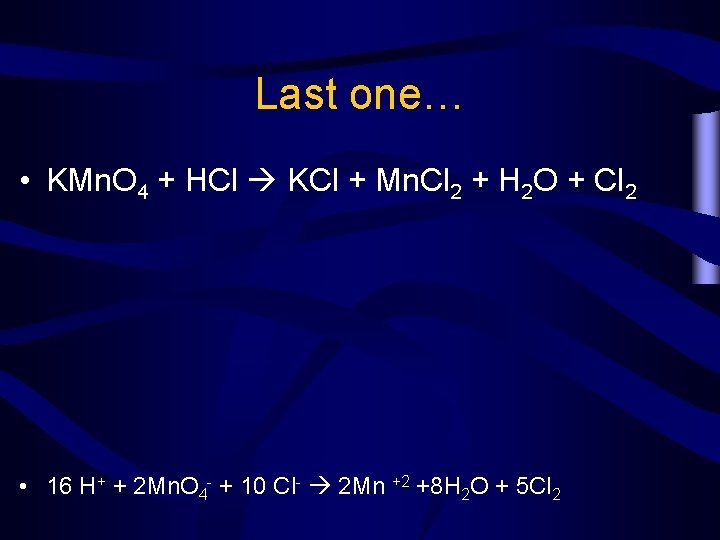
Last one… • KMn. O 4 + HCl KCl + Mn. Cl 2 + H 2 O + Cl 2 • 16 H+ + 2 Mn. O 4 - + 10 Cl- 2 Mn +2 +8 H 2 O + 5 Cl 2
- Slides: 8