BALANCING REDOX EQUATIONS USING THE HALF EQUATION METHOD

BALANCING REDOX EQUATIONS USING THE HALF EQUATION METHOD BASIC ENVIRONMENT
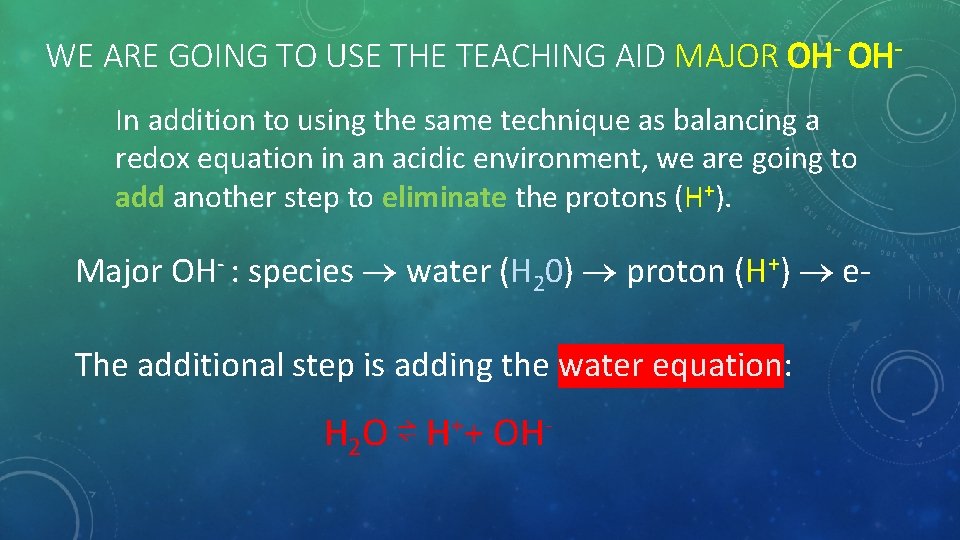
WE ARE GOING TO USE THE TEACHING AID MAJOR OH- OHIn addition to using the same technique as balancing a redox equation in an acidic environment, we are going to add another step to eliminate the protons (H+). Major OH- : species water (H 20) proton (H+) e. The additional step is adding the water equation: H 2 O ⇌ H++ OH-
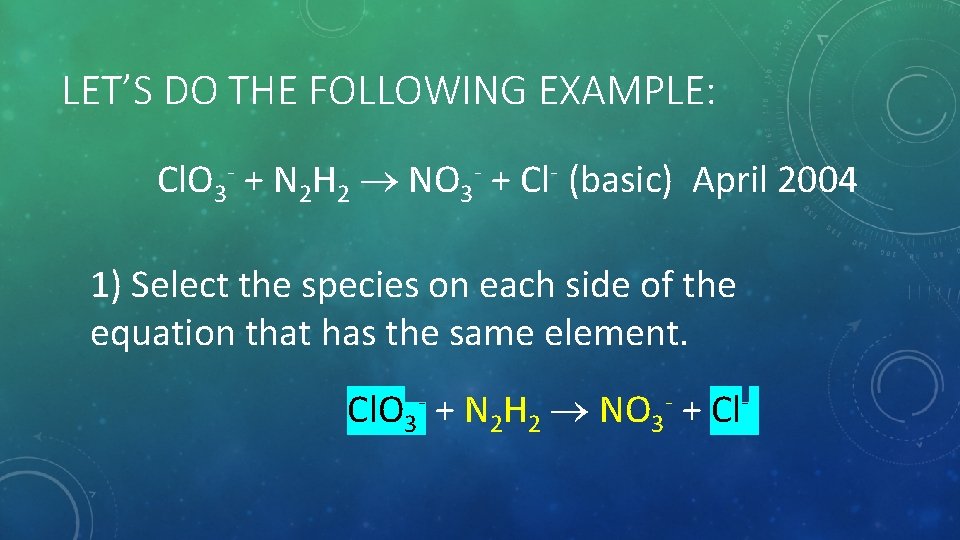
LET’S DO THE FOLLOWING EXAMPLE: Cl. O 3 - + N 2 H 2 NO 3 - + Cl- (basic) April 2004 1) Select the species on each side of the equation that has the same element. Cl. O 3 - + N 2 H 2 NO 3 - + Cl-

2) Write the two half equations: Cl. O 3 - Cl. N 2 H 2 NO 3 -

3) Let’s use Major OH- on the chlorate (Cl. O 3 -) equation: a) Major species: As there is the same number of chlorine atoms for each species, nothing is needed. Cl. O 3 - Clb) Adding H 2 O to the side having less oxygen atoms. Cl. O 3 - Cl- + 3 H 2 O
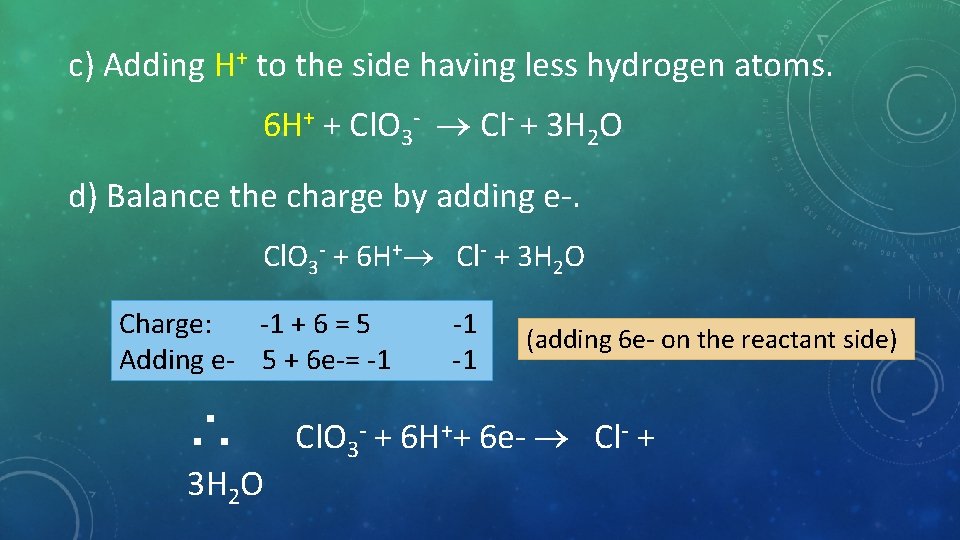
c) Adding H+ to the side having less hydrogen atoms. 6 H+ + Cl. O 3 - Cl- + 3 H 2 O d) Balance the charge by adding e-. Cl. O 3 + 6 H+ Cl + 3 H 2 O Charge: -1 + 6 = 5 Adding e- 5 + 6 e-= -1 ∴ 3 H 2 O -1 -1 (adding 6 e- on the reactant side) Cl. O 3 + 6 H++ 6 e- Cl +

4) LET’S USE MAJOR OH- WITH THE HYDRAZINE (N 2 H 2) EQUATION: N 2 H 2 NO 3 a) Major species: There are more nitrogen atoms on the reactant side, so that we need to add a coefficient to the product side. N 2 H 2 2 NO 3 -

b) Adding H 2 O to the side having less oxygen atoms. As there are 6 atoms of oxygen on the product side, we need to add 6 H 2 O on the reactant side. N 2 H 2 + 6 H 2 O 2 NO 3 c) Adding hydrogen ion (H+) to the side having less hydrogen atoms. As there are 14 hydrogen atoms on the reactant side, we need to add 14 H+ on the product side. N 2 H 2 + 6 H 2 O 2 NO 3 - + 16 H+

d) Balancing the charge by adding electrons(e ) to the side that is more positive. N 2 H 2 + 6 H 2 O 2 NO 3 - + 16 H+ Charge: Adding e- 0 0 ∴ 14 e- -2 +16 = +14 + 14 e- = 0 N 2 H 2 + 6 H 2 O 2 NO 3 - + 16 H+ +

4) LET’S RECORD THE TWO COMPLETED HALF EQUATIONS: Cl. O 3 + 6 H+ + 6 e- Cl + 3 H 2 O (reduction reaction) N 2 H 2 + 6 H 2 O 2 NO 3 - + 16 H+ + 14 e- (oxidation reaction)

5) BEFORE WE ADD THESE TWO EQUATIONS AND WE NEED TO MULTIPLY THESE BY A COEFFICIENT SO THAT BOTH EQUATIONS HAVE THE SAME NUMBER OF ELECTRONS ON OPPOSITE SIDES. (Cl. O 3 + 6 H+ + 6 e- Cl + 3 H 2 O) X 7 (N 2 H 2 + 6 H 2 O 2 NO 3 - + 16 H+ + 14 e-) X 3

6) LET’S RE-WRITE THESE TWO EQUATIONS WITH THE SAME NUMBER OF ELECTRONS ON OPPOSITE SIDE. 7 Cl. O 3 + 42 H+ + 42 e- 7 Cl + 21 H 2 O 3 N 2 H 2 + 18 H 2 O 6 NO 3 - + 48 H+ + 42 e-

7) WE ADD THESE TWO EQUATIONS TO CANCEL OUT THE NUMBER OF ELECTRONS (e-) 7 Cl. O 3 + 42 H+ +3 N 2 H 2 + 18 H 2 O 6 NO 3 - + 48 H+ + 7 Cl + 21 H 2 O

8) WE REDUCE THE NUMBER OF H 2 O AS WELL AS THE NUMBER OF H+: 7 Cl. O 3 +3 N 2 H 2 6 NO 3 - + 6 H+ + 7 Cl + 3 H 2 O

9) THEN, WE WRITE THE WATER EQUATION SO THAT THE H+ CANCEL OUT: 7 Cl. O 3 +3 N 2 H 2 6 NO 3 - + 6 H+ + 7 Cl + 3 H 2 O 6 H+ + 6 OH ⇌ 6 H 2 O

10) LAST, WE ADD THESE TWO EQUATIONS AND WE ADD UP THE WATER (H 2 O) 7 Cl. O 3 +3 N 2 H 2 + 6 OH ⇌ 6 NO 3 - + 7 Cl + 9 H 2 O
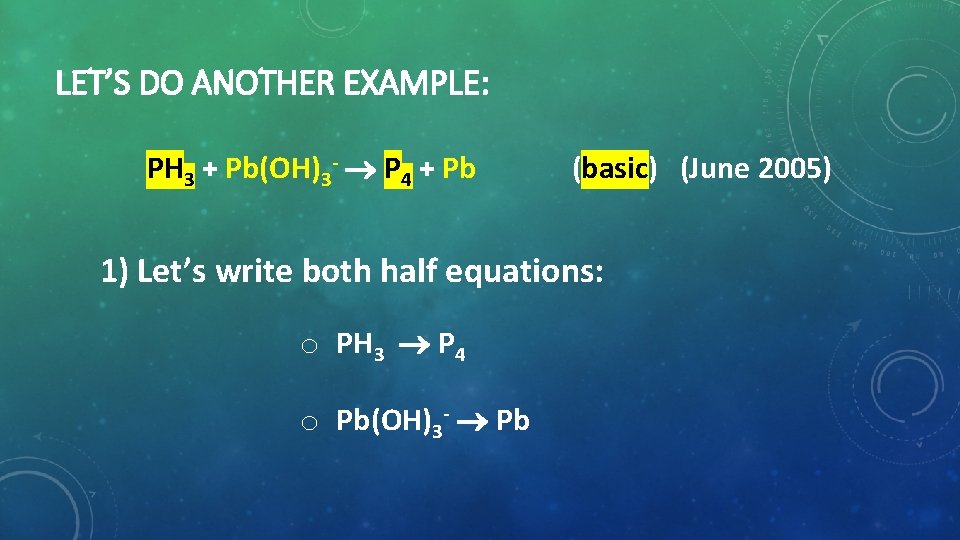
LET’S DO ANOTHER EXAMPLE: PH 3 + Pb(OH)3 P 4 + Pb (basic) (June 2005) 1) Let’s write both half equations: o PH 3 P 4 o Pb(OH)3 Pb
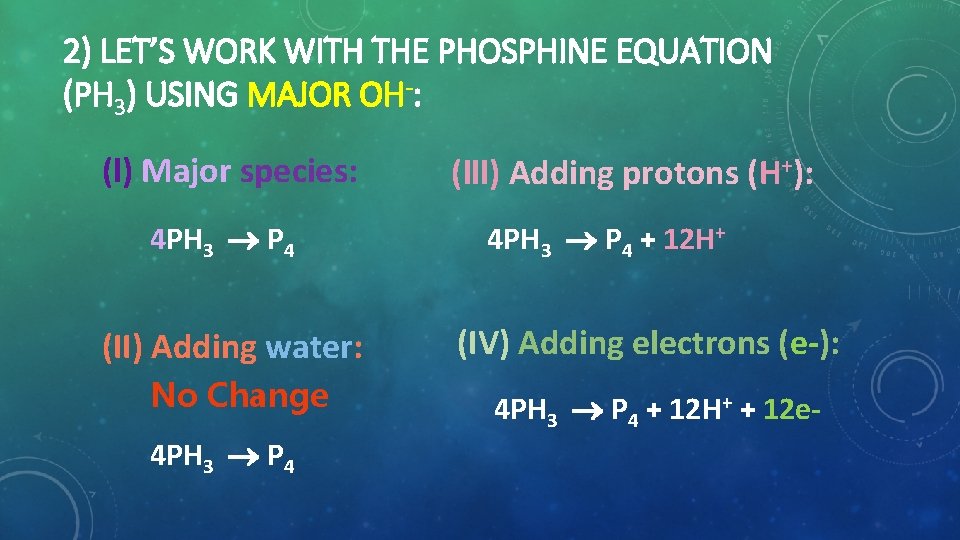
2) LET’S WORK WITH THE PHOSPHINE EQUATION (PH 3) USING MAJOR OH-: (l) Major species: 4 PH 3 P 4 (lll) Adding protons (H+): 4 PH 3 P 4 + 12 H+ (II) Adding water: (IV) Adding electrons (e ): No Change 4 PH 3 P 4 + 12 H+ + 12 e 4 PH 3 P 4
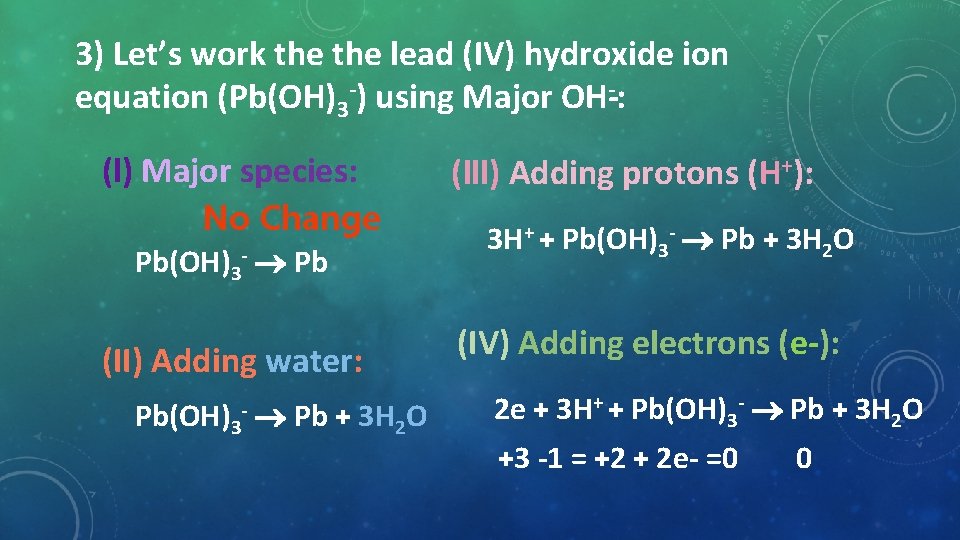
3) Let’s work the lead (IV) hydroxide ion equation (Pb(OH)3 ) using Major OH : (l) Major species: No Change Pb(OH)3 Pb (II) Adding water: Pb(OH)3 Pb + 3 H 2 O (lll) Adding protons (H+): 3 H+ + Pb(OH)3 Pb + 3 H 2 O (IV) Adding electrons (e ): 2 e + 3 H+ + Pb(OH)3 Pb + 3 H 2 O +3 1 = +2 + 2 e =0 0

4) MULTIPLY BY A NUMBER SO THAT BOTH EQUATIONS HAVE THE SAME NUMBER OF ELECTRONS: 4 PH 3 P 4 + 12 H+ + 12 e (2 e + 3 H+ + Pb(OH)3 Pb + 3 H 2 O) X 6

5) ADD BOTH EQUATIONS, CANCELLING ELECTRONS AND REDUCING PROTONS: + 6 H + 6 Pb(OH)3 + 4 PH 3 P 4 + 6 Pb + 18 H 2 O
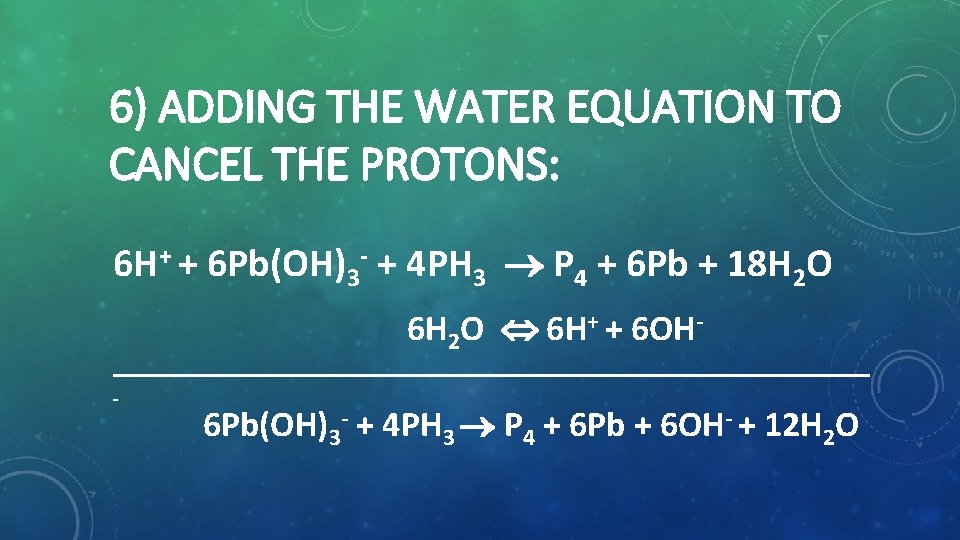
6) ADDING THE WATER EQUATION TO CANCEL THE PROTONS: 6 H+ + 6 Pb(OH)3 + 4 PH 3 P 4 + 6 Pb + 18 H 2 O 6 H 2 O 6 H+ + 6 OH 6 Pb(OH)3 + 4 PH 3 P 4 + 6 Pb + 6 OH + 12 H 2 O
- Slides: 22