BALANCING REDOX EQUATIONS To balance a redox equation
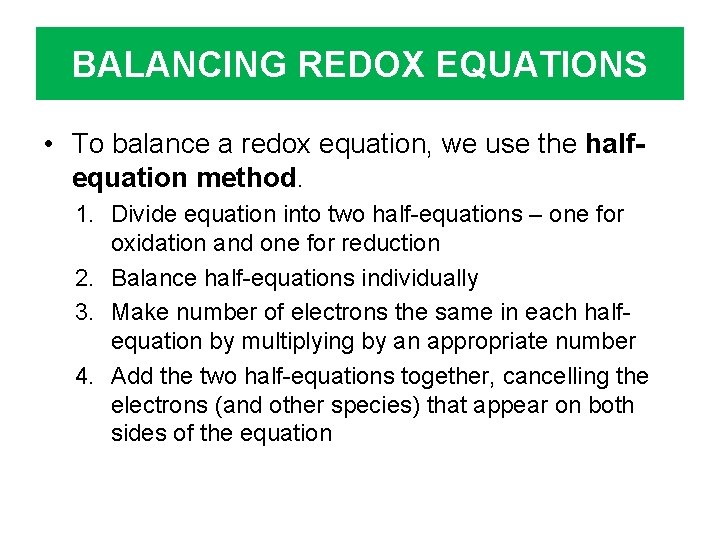
BALANCING REDOX EQUATIONS • To balance a redox equation, we use the halfequation method. 1. Divide equation into two half-equations – one for oxidation and one for reduction 2. Balance half-equations individually 3. Make number of electrons the same in each halfequation by multiplying by an appropriate number 4. Add the two half-equations together, cancelling the electrons (and other species) that appear on both sides of the equation
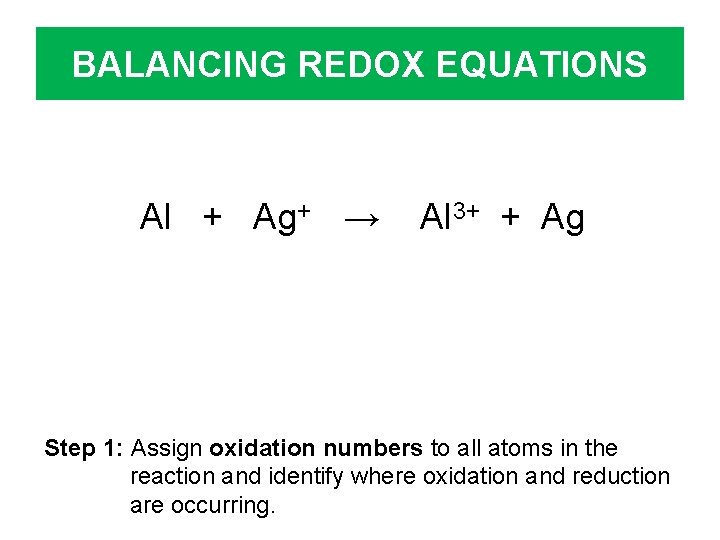
BALANCING REDOX EQUATIONS Al + Ag+ → Al 3+ + Ag Step 1: Assign oxidation numbers to all atoms in the reaction and identify where oxidation and reduction are occurring.
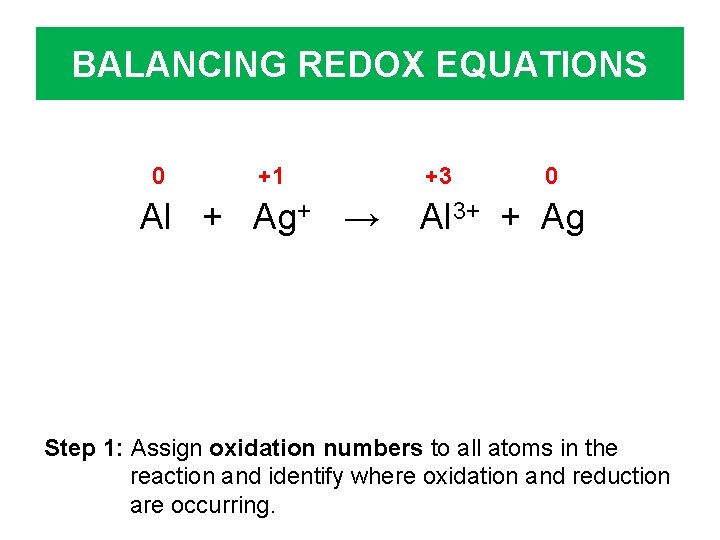
BALANCING REDOX EQUATIONS 0 +1 Al + Ag+ → +3 0 Al 3+ + Ag Step 1: Assign oxidation numbers to all atoms in the reaction and identify where oxidation and reduction are occurring.
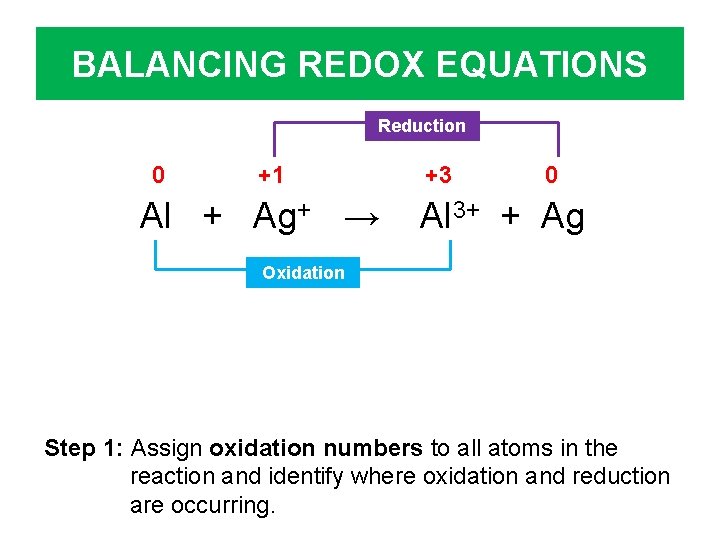
BALANCING REDOX EQUATIONS Reduction 0 +1 Al + Ag+ → +3 0 Al 3+ + Ag Oxidation Step 1: Assign oxidation numbers to all atoms in the reaction and identify where oxidation and reduction are occurring.
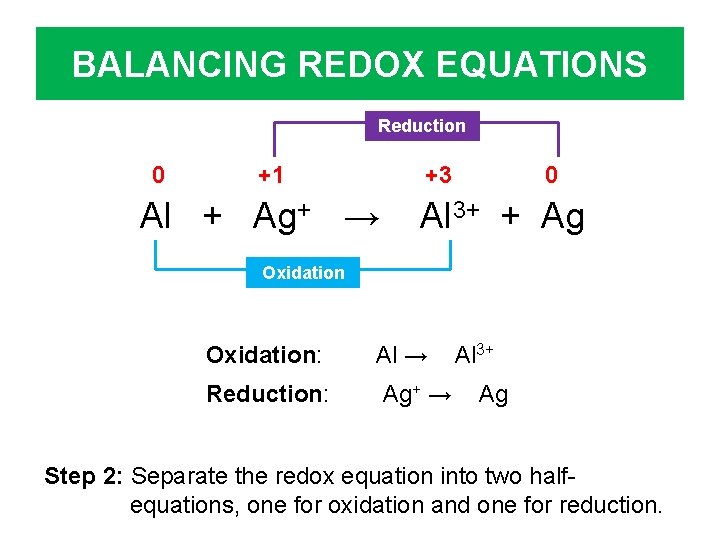
BALANCING REDOX EQUATIONS Reduction 0 +1 +3 Al + Ag+ → 0 Al 3+ + Ag Oxidation: Al → Reduction: Ag+ → Al 3+ Ag Step 2: Separate the redox equation into two halfequations, one for oxidation and one for reduction.
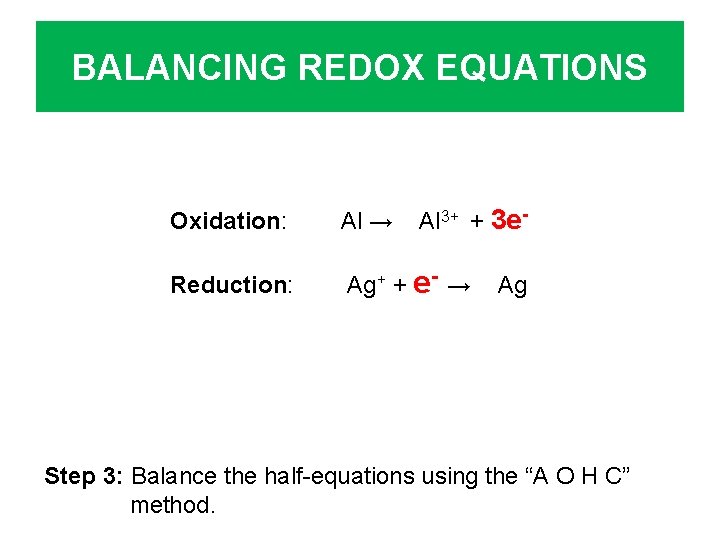
BALANCING REDOX EQUATIONS Al 3+ + 3 e- Oxidation: Al → Reduction: Ag+ + e- → Ag Step 3: Balance the half-equations using the “A O H C” method.
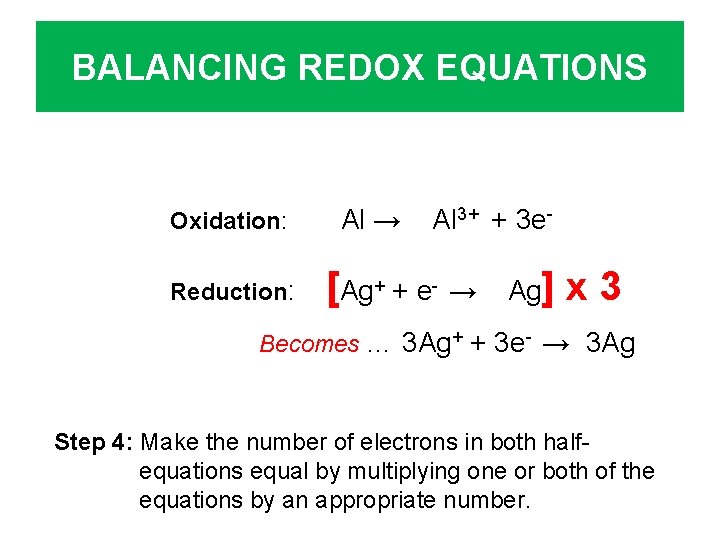
BALANCING REDOX EQUATIONS Oxidation: Reduction: Al → Al 3+ + 3 e- [Ag+ + e- → Ag] x 3 Becomes … 3 Ag+ + 3 e- → 3 Ag Step 4: Make the number of electrons in both halfequations equal by multiplying one or both of the equations by an appropriate number.
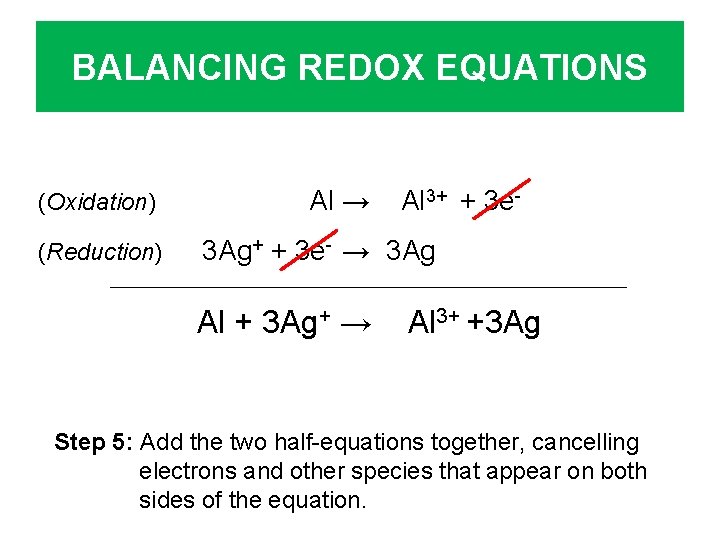
BALANCING REDOX EQUATIONS (Oxidation) (Reduction) Al → Al 3+ + 3 e- 3 Ag+ + 3 e- → 3 Ag Al + 3 Ag+ → Al 3+ +3 Ag Step 5: Add the two half-equations together, cancelling electrons and other species that appear on both sides of the equation.
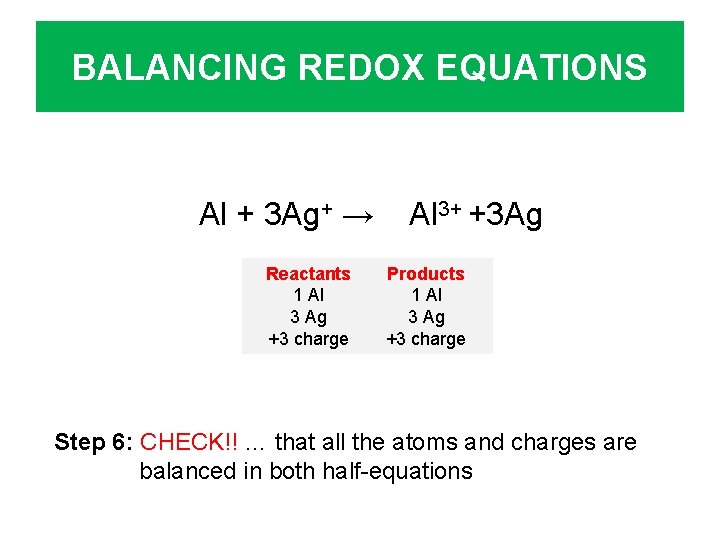
BALANCING REDOX EQUATIONS Al + 3 Ag+ → Reactants 1 Al 3 Ag +3 charge Al 3+ +3 Ag Products 1 Al 3 Ag +3 charge Step 6: CHECK!! … that all the atoms and charges are balanced in both half-equations
- Slides: 9