Balancing Nuclear Equations In a balanced nuclear equation
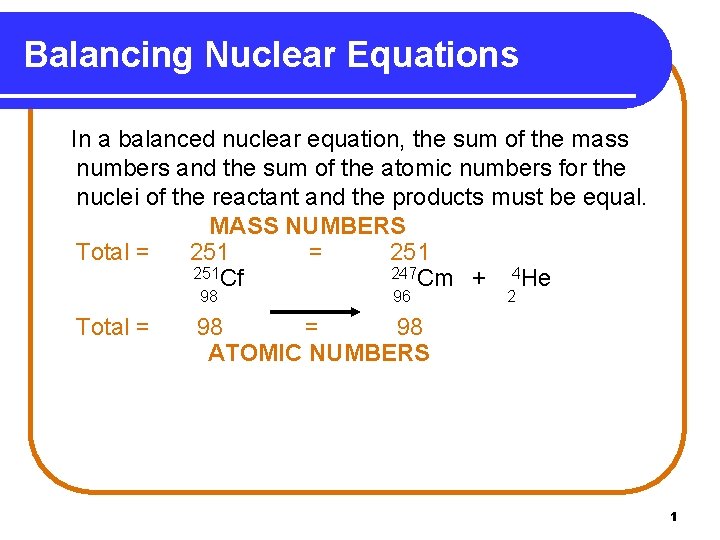
Balancing Nuclear Equations In a balanced nuclear equation, the sum of the mass numbers and the sum of the atomic numbers for the nuclei of the reactant and the products must be equal. MASS NUMBERS Total = 251 251 Cf 247 Cm + 4 He 98 Total = 96 2 98 = 98 ATOMIC NUMBERS 1
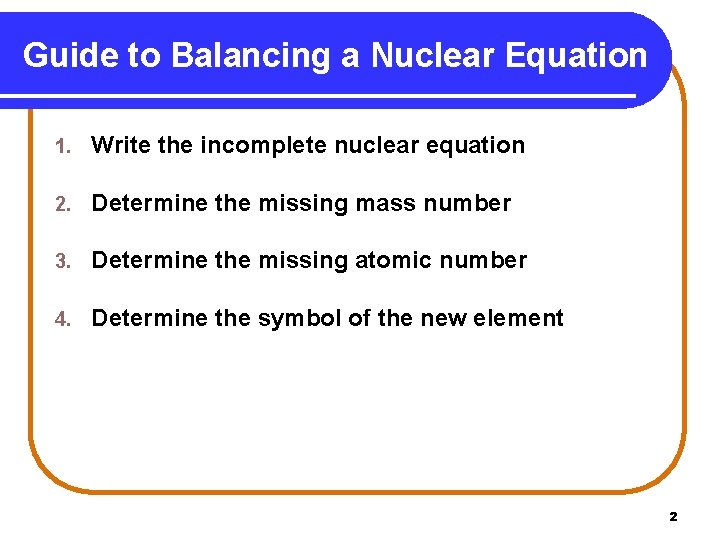
Guide to Balancing a Nuclear Equation 1. Write the incomplete nuclear equation 2. Determine the missing mass number 3. Determine the missing atomic number 4. Determine the symbol of the new element 2
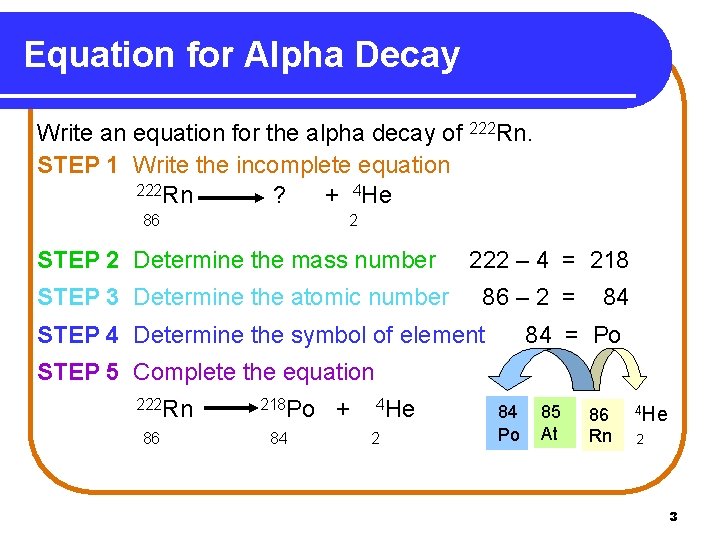
Equation for Alpha Decay Write an equation for the alpha decay of 222 Rn. STEP 1 Write the incomplete equation 222 Rn ? s + 4 He 86 2 STEP 2 Determine the mass number STEP 3 Determine the atomic number 222 – 4 = 218 86 – 2 = STEP 4 Determine the symbol of element 84 84 = Po STEP 5 Complete the equation 222 Rn 86 218 Po 84 + 4 He 2 84 Po 85 At 86 Rn 4 He 2 3
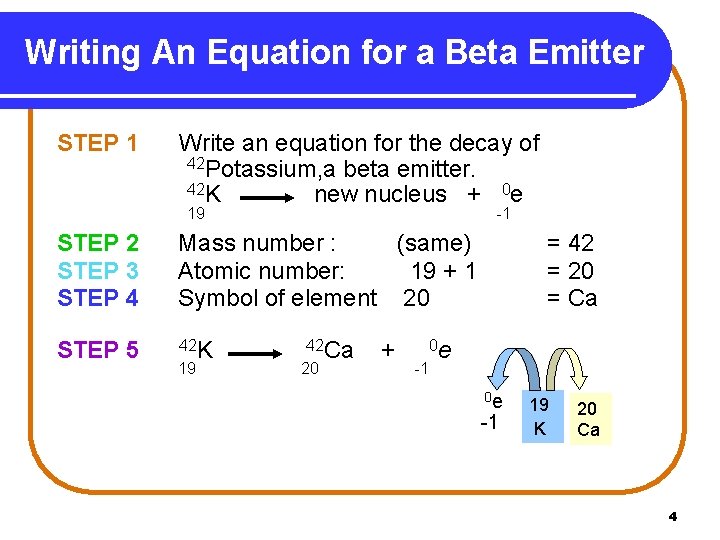
Writing An Equation for a Beta Emitter STEP 1 Write an equation for the decay of 42 Potassium, a beta emitter. 42 K new nucleus + 0 e 19 -1 STEP 2 STEP 3 STEP 4 Mass number : (same) Atomic number: 19 + 1 Symbol of element 20 STEP 5 42 K 19 42 Ca 20 + = 42 = 20 = Ca 0 e -1 19 K 20 Ca 4
Learning Check Write the nuclear equation for the beta decay of 60 Co. 5
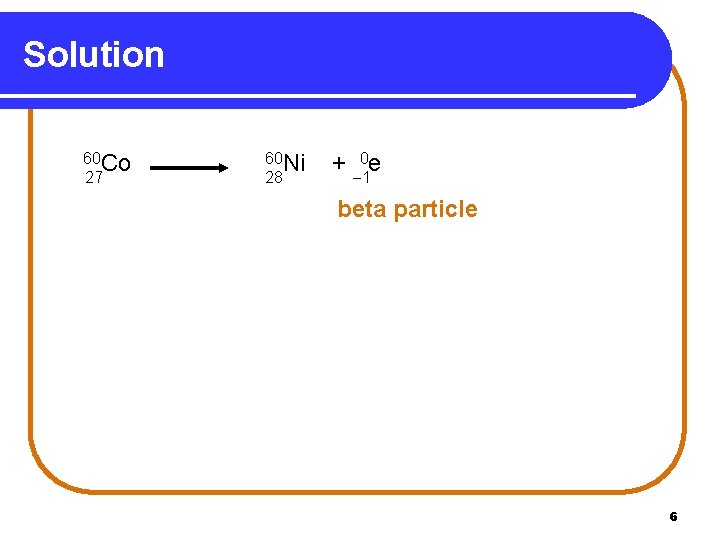
Solution 60 Co 27 60 Ni 28 + 0 e 1 beta particle 6
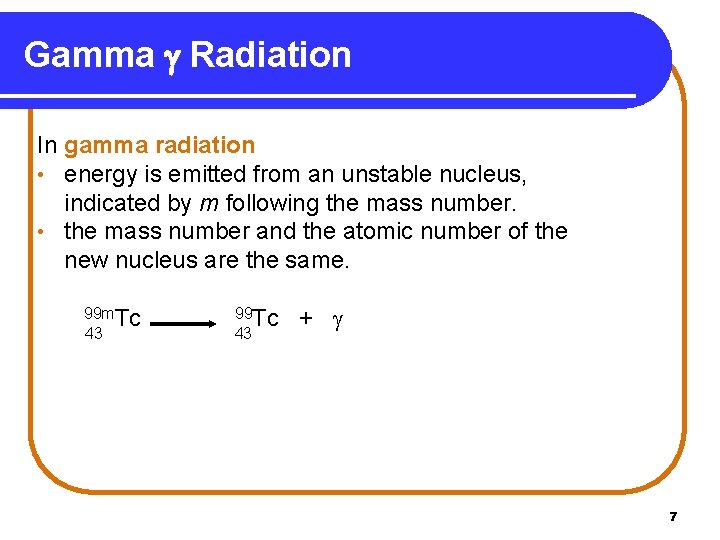
Gamma Radiation In gamma radiation • energy is emitted from an unstable nucleus, indicated by m following the mass number. • the mass number and the atomic number of the new nucleus are the same. 99 m. Tc 43 99 Tc 43 + 7
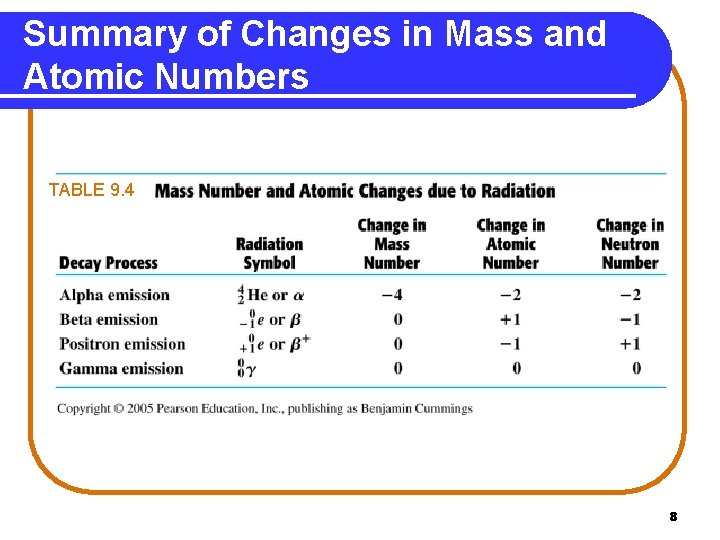
Summary of Changes in Mass and Atomic Numbers TABLE 9. 4 8
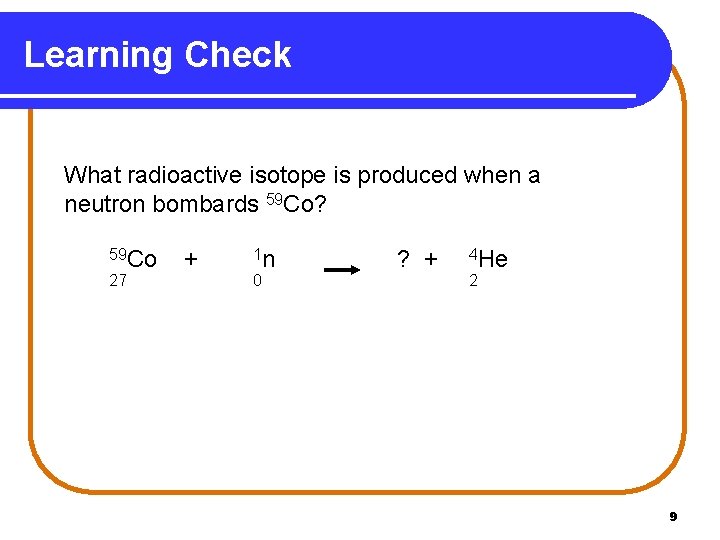
Learning Check What radioactive isotope is produced when a neutron bombards 59 Co? 59 Co 27 + 1 n 0 ? + 4 He 2 9
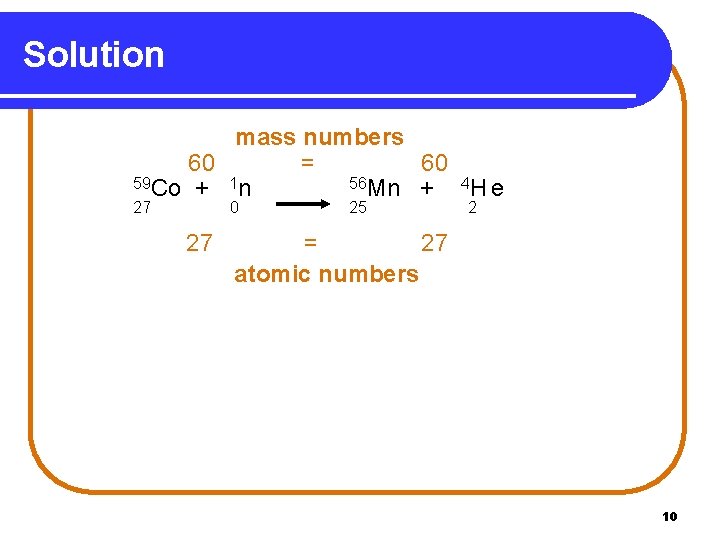
Solution mass numbers 60 = 60 59 Co + 1 n 56 Mn + 4 H e 27 0 27 25 2 = 27 atomic numbers 10
- Slides: 10