Balancing Equations What is balancing an equation Balancing

Balancing Equations
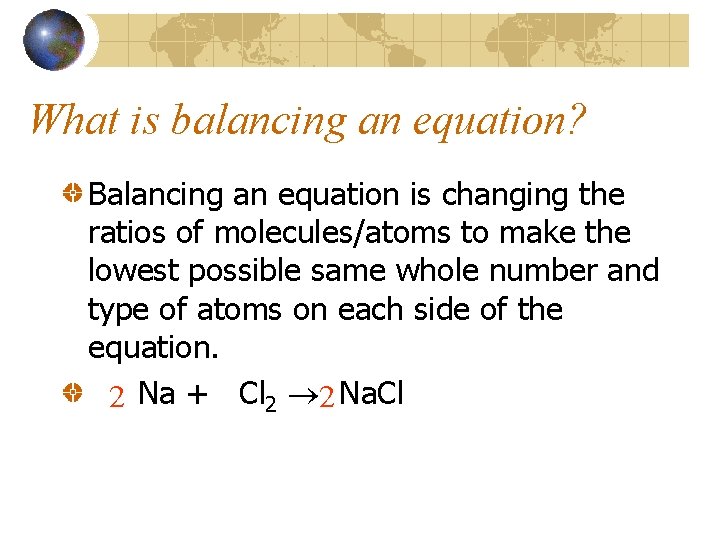
What is balancing an equation? Balancing an equation is changing the ratios of molecules/atoms to make the lowest possible same whole number and type of atoms on each side of the equation. 2 Na + Cl 2 2 Na. Cl
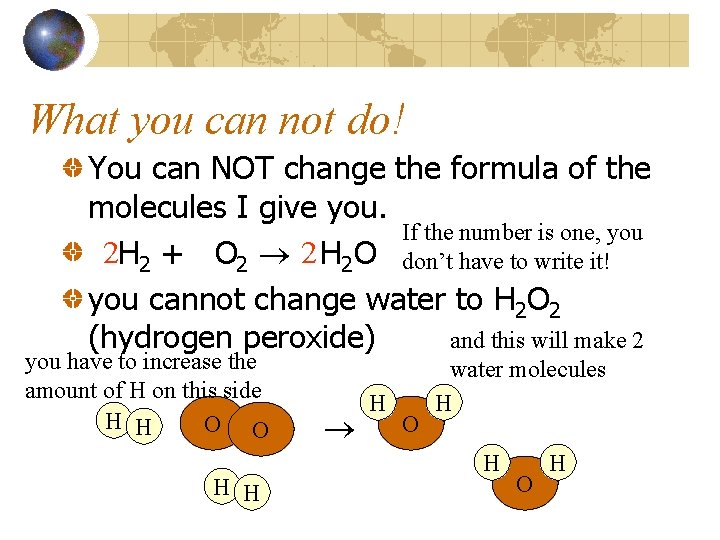
What you can not do! You can NOT change the formula of the molecules I give you. If the number is one, you 2 H 2 + O 2 2 H 2 O don’t have to write it! you cannot change water to H 2 O 2 and this will make 2 (hydrogen peroxide) you have to increase the amount of H on this side H H O O H H H O water molecules H H O H

What you are allowed to do change the coefficient in front of any molecule/atom (changing the ratio of atoms) 2 Ag + ___ Cl ___ 2 Ag. Cl ___ 2 you can only write on those lines

Problem solving strategies It helps to make a list of all atoms present 2 HCl + Ba(OH)2 Ba. Cl 2 + 2 H 2 O Ba – 1 H– 3 4 Cl – 1 2 O– 2 starting w/ Cl Start making the Ba – 1 numbers agree H– 2 4 one at a time (pick something, Cl – 2 that occurs in only O – 1 2 one molecule 1 st) ~not H in this case Now going to O Now everything is balanced!
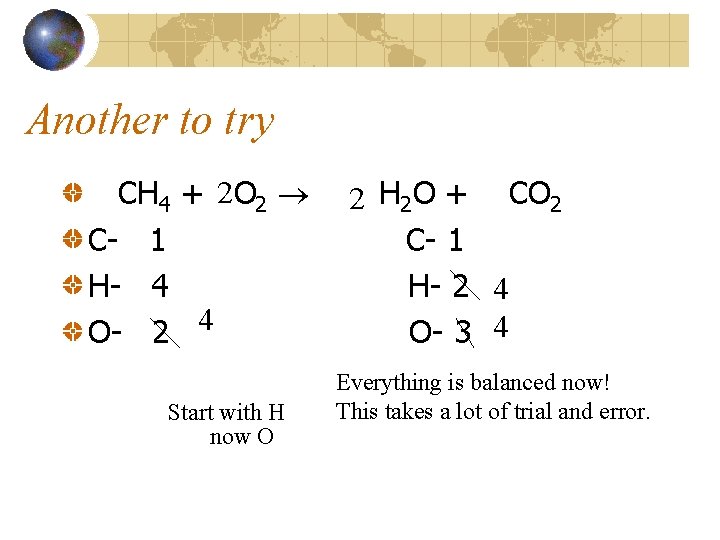
Another to try CH 4 + 2 O 2 C- 1 H- 4 O- 2 4 Start with H now O 2 H 2 O + CO 2 C- 1 H- 2 4 O- 3 4 Everything is balanced now! This takes a lot of trial and error.
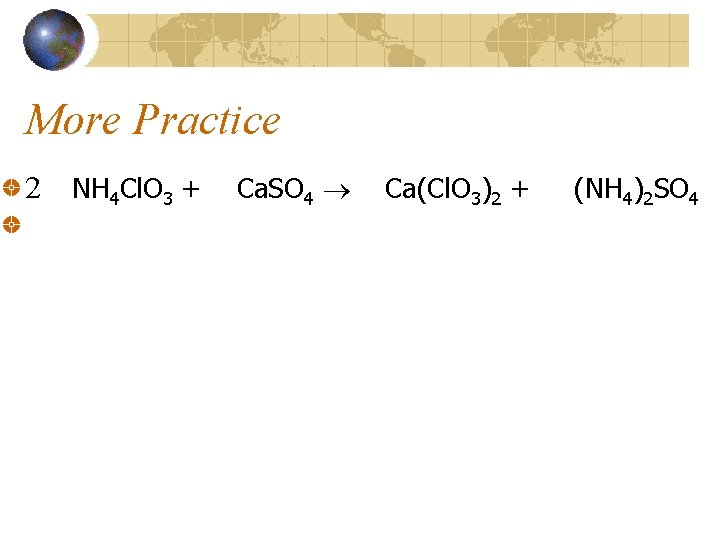
More Practice 2 NH 4 Cl. O 3 + Ca. SO 4 Ca(Cl. O 3)2 + (NH 4)2 SO 4
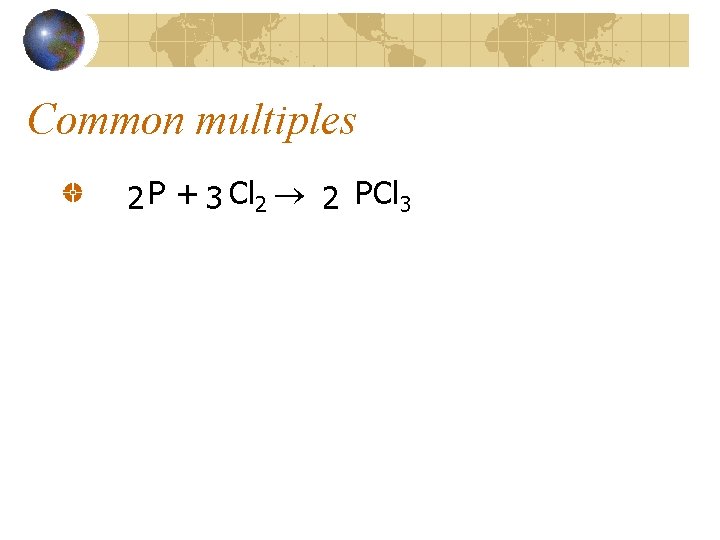
Common multiples 2 P + 3 Cl 2 2 PCl 3
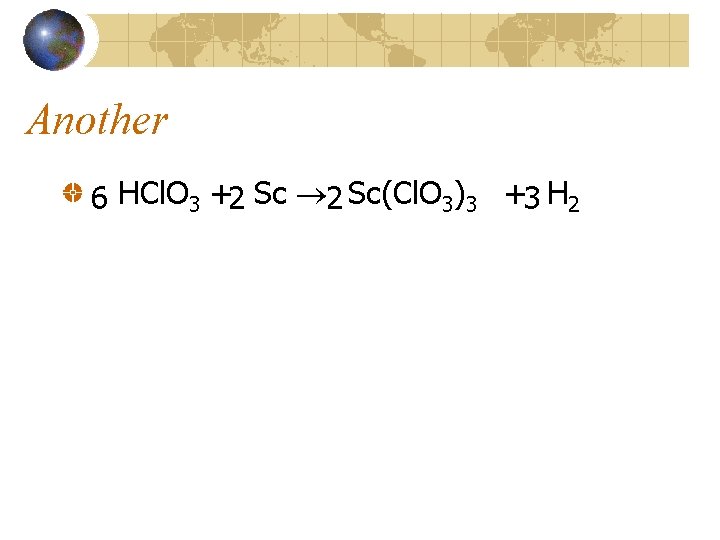
Another 6 HCl. O 3 +2 Sc(Cl. O 3)3 +3 H 2

Homework HNO 3 + Zn H 2 + Zn. NO 3
- Slides: 10