Balancing Equations Chemical Equations are concise representations of

Balancing Equations
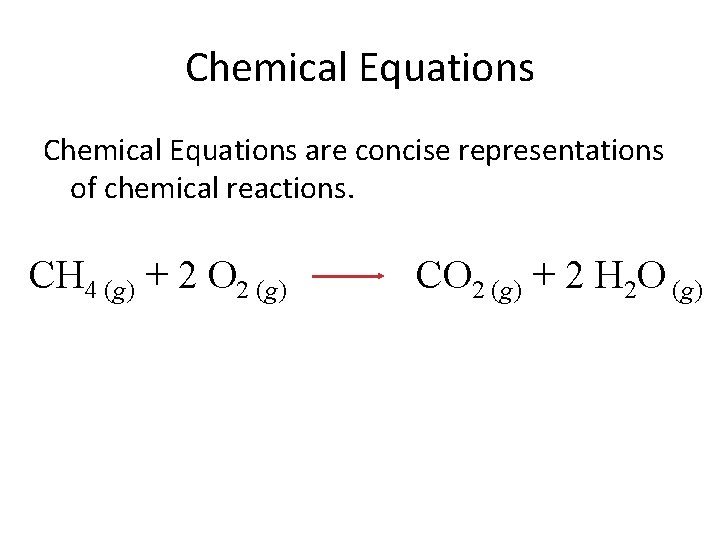
Chemical Equations are concise representations of chemical reactions. CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g)
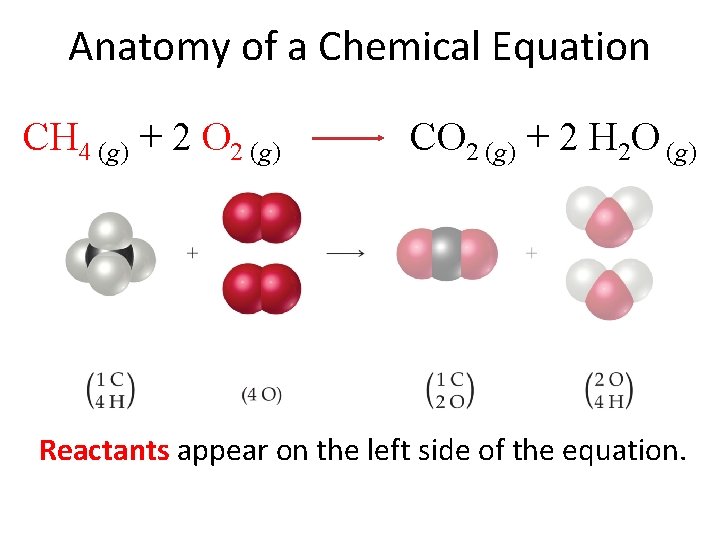
Anatomy of a Chemical Equation CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g) Reactants appear on the left side of the equation.
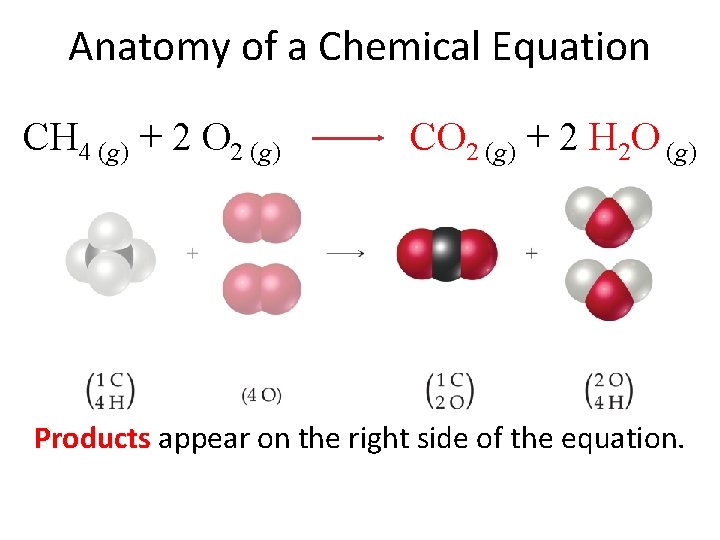
Anatomy of a Chemical Equation CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g) Products appear on the right side of the equation.
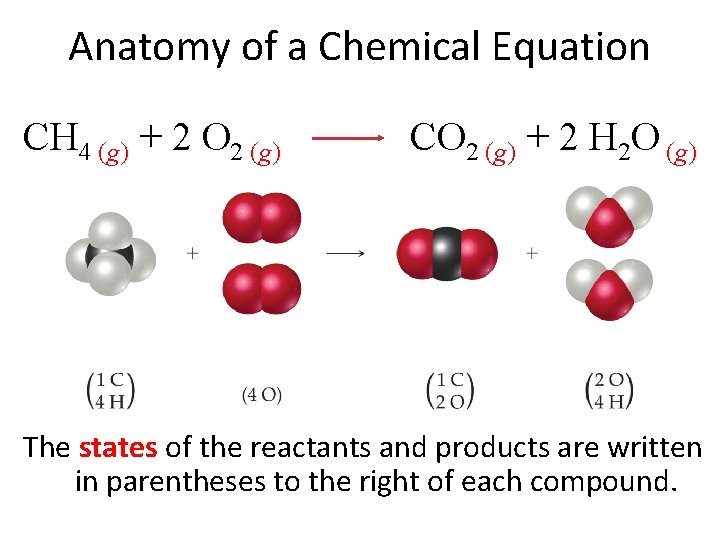
Anatomy of a Chemical Equation CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g) The states of the reactants and products are written in parentheses to the right of each compound.
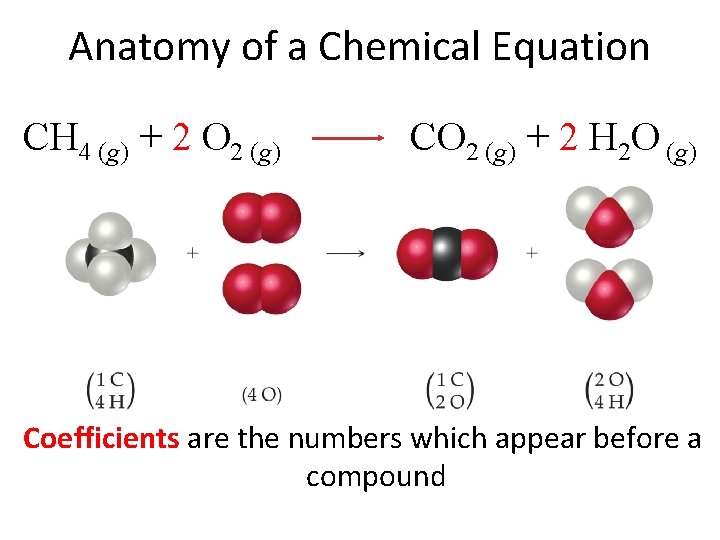
Anatomy of a Chemical Equation CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g) Coefficients are the numbers which appear before a compound
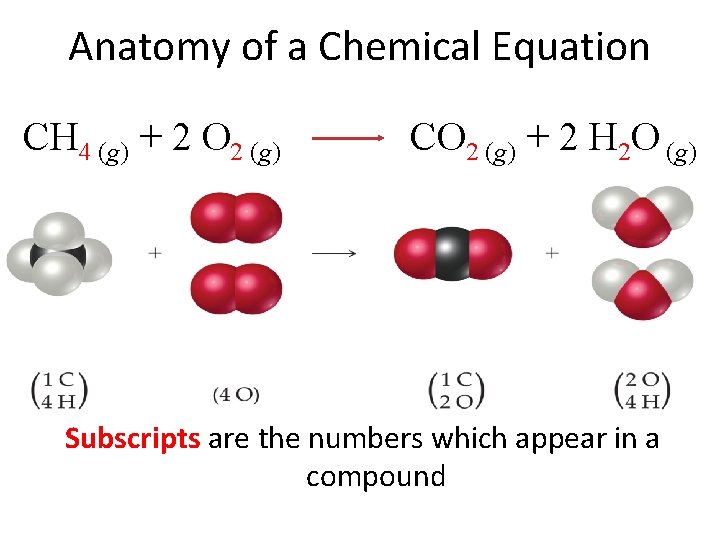
Anatomy of a Chemical Equation CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g) Subscripts are the numbers which appear in a compound
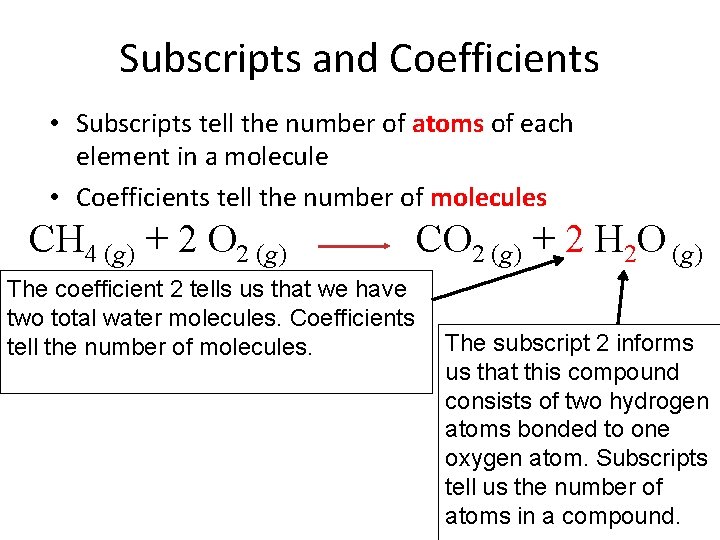
Subscripts and Coefficients • Subscripts tell the number of atoms of each element in a molecule • Coefficients tell the number of molecules CH 4 (g) + 2 O 2 (g) The coefficient 2 tells us that we have two total water molecules. Coefficients tell the number of molecules. CO 2 (g) + 2 H 2 O (g) The subscript 2 informs us that this compound consists of two hydrogen atoms bonded to one oxygen atom. Subscripts tell us the number of atoms in a compound.
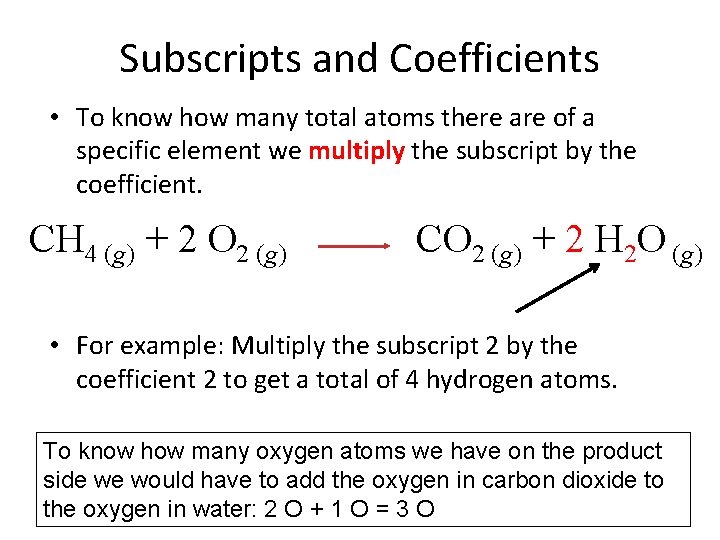
Subscripts and Coefficients • To know how many total atoms there are of a specific element we multiply the subscript by the coefficient. CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g) • For example: Multiply the subscript 2 by the coefficient 2 to get a total of 4 hydrogen atoms. To know how many oxygen atoms we have on the product side we would have to add the oxygen in carbon dioxide to the oxygen in water: 2 O + 1 O = 3 O
Balancing Equations
Balancing Equations • Law of Conservation of Matter: – In a chemical reaction, matter can be neither created nor destroyed.
Balancing Equations • In other words: – The number of atoms of each type of element must be the same on each side of the equation.
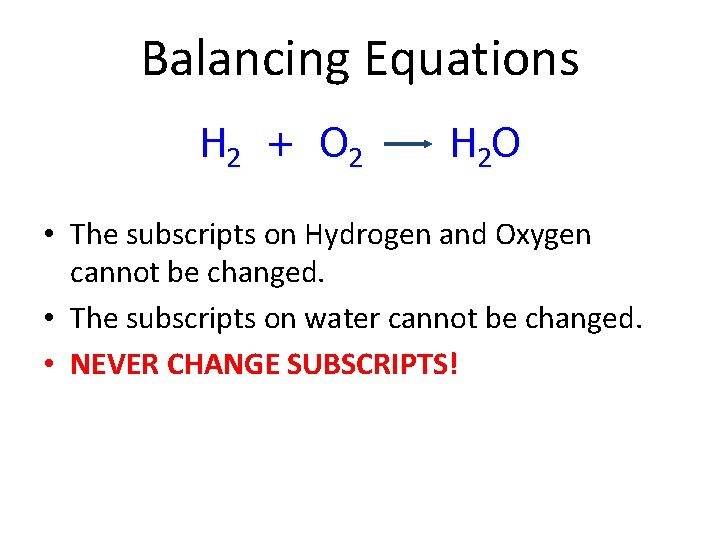
Balancing Equations H 2 + O 2 H 2 O • The subscripts on Hydrogen and Oxygen cannot be changed. • The subscripts on water cannot be changed. • NEVER CHANGE SUBSCRIPTS!
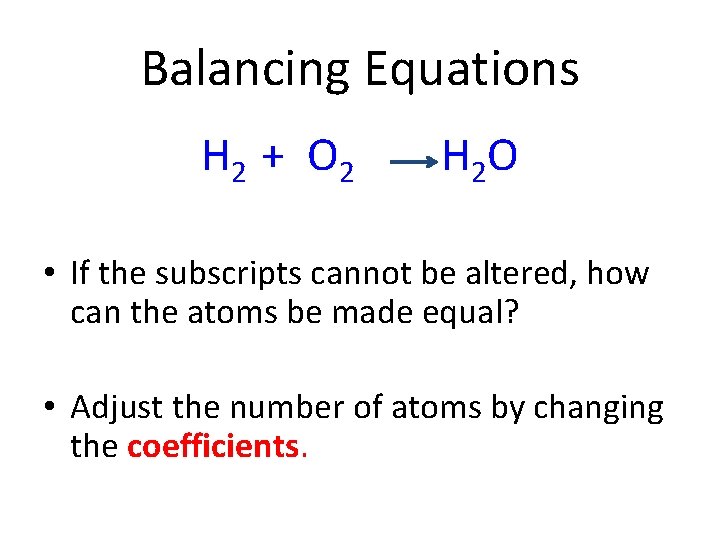
Balancing Equations H 2 + O 2 H 2 O • If the subscripts cannot be altered, how can the atoms be made equal? • Adjust the number of atoms by changing the coefficients.
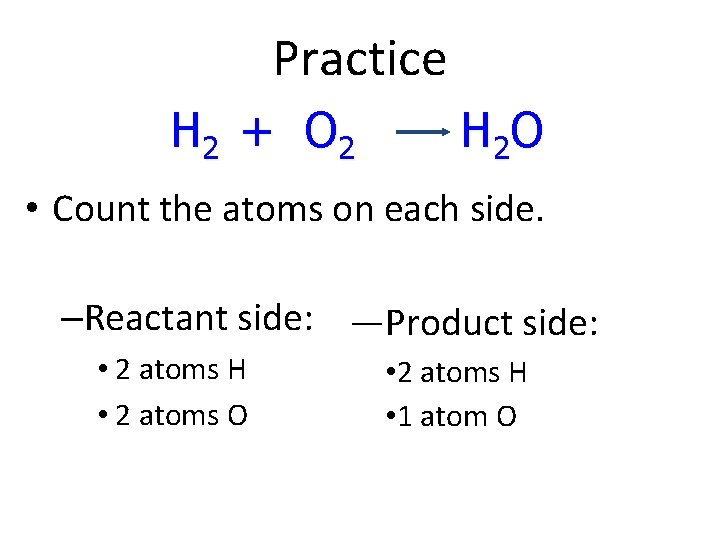
Practice H 2 + O 2 H 2 O • Count the atoms on each side. –Reactant side: —Product side: • 2 atoms H • 2 atoms O • 2 atoms H • 1 atom O
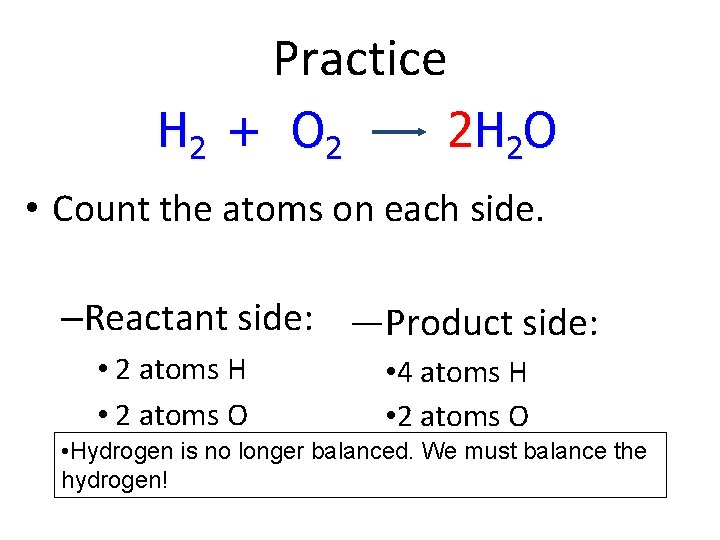
Practice H 2 + O 2 2 H 2 O • Count the atoms on each side. –Reactant side: —Product side: • 2 atoms H • 2 atoms O • 4 atoms H • 2 atoms O • Hydrogen is no longer balanced. We must balance the hydrogen!
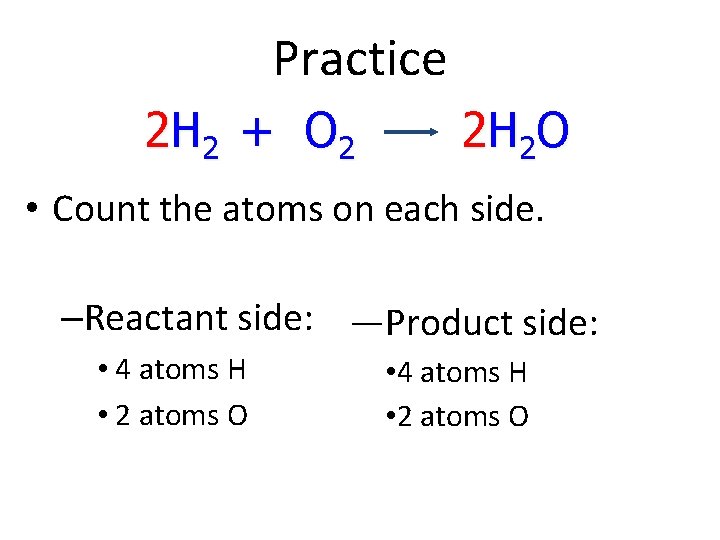
Practice 2 H 2 + O 2 2 H 2 O • Count the atoms on each side. –Reactant side: —Product side: • 4 atoms H • 2 atoms O
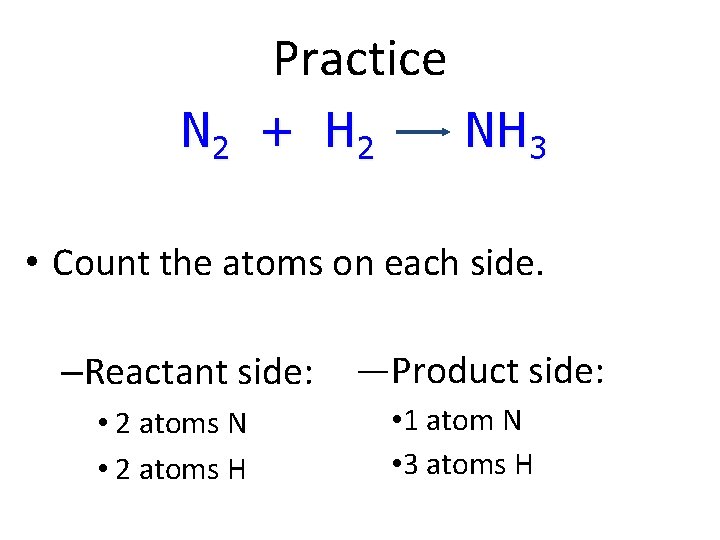
Practice N 2 + H 2 NH 3 • Count the atoms on each side. –Reactant side: • 2 atoms N • 2 atoms H —Product side: • 1 atom N • 3 atoms H
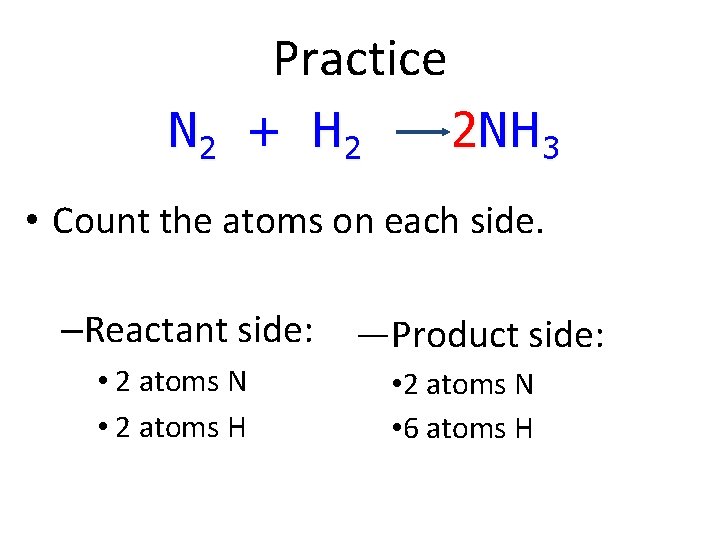
Practice N 2 + H 2 2 NH 3 • Count the atoms on each side. –Reactant side: • 2 atoms N • 2 atoms H —Product side: • 2 atoms N • 6 atoms H
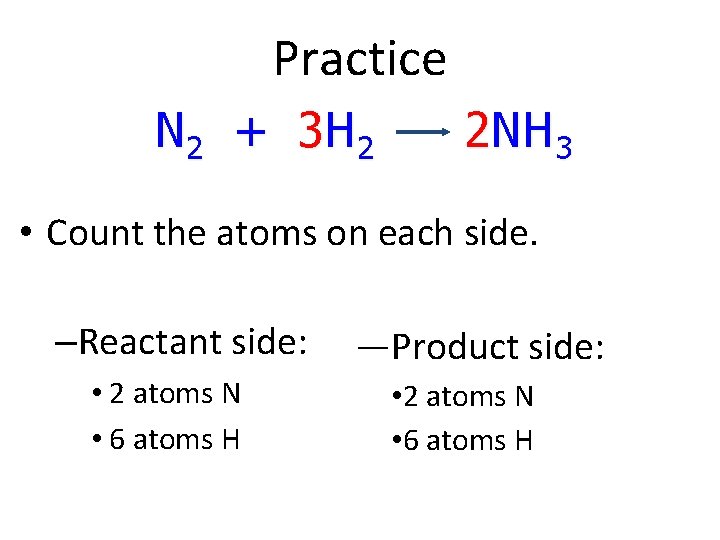
Practice N 2 + 3 H 2 2 NH 3 • Count the atoms on each side. –Reactant side: • 2 atoms N • 6 atoms H —Product side: • 2 atoms N • 6 atoms H
- Slides: 20