Balancing Equations Are you up for the challenge

Balancing Equations Are you up for the challenge?

Hello Be Proactive u Take out the supplies you need: – – u Science Notebook Pencil Homework Science Folder Everything else gets put away. Bell Work u Open your science notebook to the next available page. Title that page “Balancing Equations. ” u What is the law of conservation of mass?

Why do you need to balance equations? u The Law of Conservation of Mass says that the mass of the reactants must equal the mass of the products. u In this example: H 2 + O 2 ---> H 2 O There are 2 hydrogen’s on the left and 2 Oxygen’s, but there is only 1 oxygen on the right.

H 2 + O 2 ---> H 2 O The reactants do not equal the products! u Where did the extra oxygen go? u Atoms can’t be created nor destroyed. u They didn’t go anywhere, you just need to add numbers so the sides become equal u An equation is balanced by changing coefficients in a somewhat trial-and-error fashion u

Important Vocabulary- Coefficient u Coefficient- Large number located in front of an element or compound in a chemical formula. 2 H= 2 hydrogen's u 4 H 20 u. Coefficient is 4 u. H 2 O H 2 O

Important Vocabulary- Subscript u Subscript- The small number that is in the lower right corner of an element u. H 2 0 u Subscript is 2 u 2 hydrogen’s H H

u If there is no coefficient or subscriptassume it is 1 1 C 1 S 2 u. Coefficient=1 u. C’s subscript= 1 u. S’s subscript= 2

Let’s practice u 2 Na 2 O u 4 H 3 PO 4 5 H 2 O 2 u 3 S 8 u 6 K 20 u
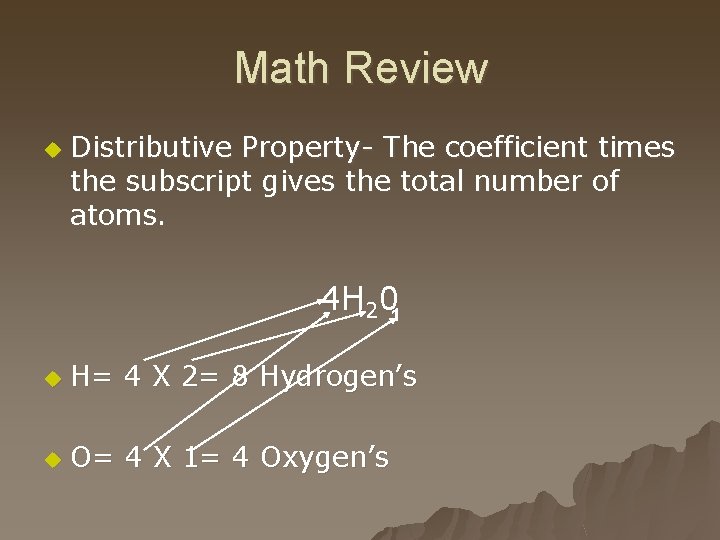
Math Review u Distributive Property- The coefficient times the subscript gives the total number of atoms. 4 H 201 u H= 4 X 2= 8 Hydrogen’s u O= 4 X 1= 4 Oxygen’s
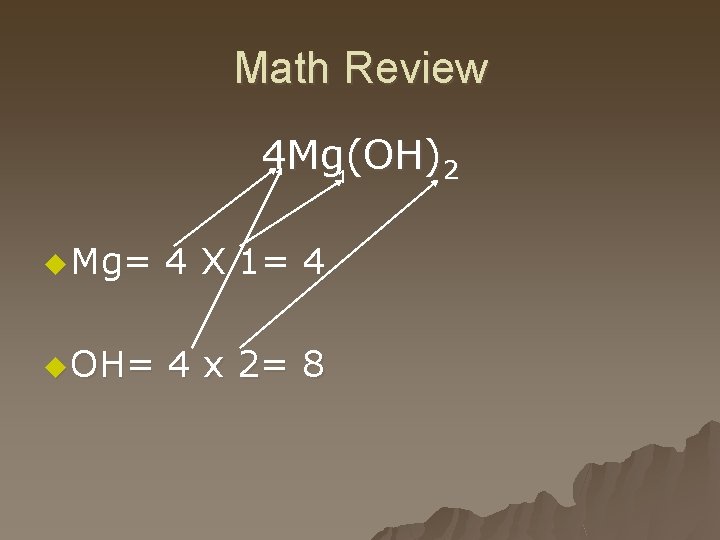
Math Review 4 Mg(OH) 2 1 u Mg= 4 X 1= 4 u OH= 4 x 2= 8
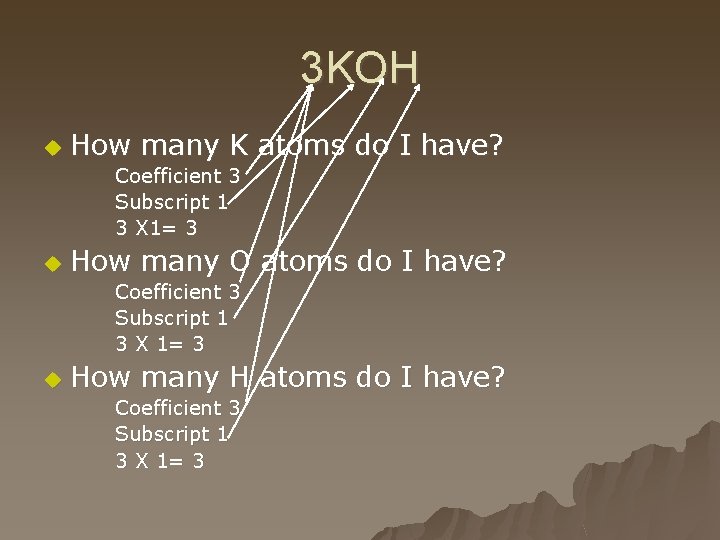
3 KOH u How many K atoms do I have? Coefficient 3 Subscript 1 3 X 1= 3 u How many O atoms do I have? Coefficient 3 Subscript 1 3 X 1= 3 u How many H atoms do I have? Coefficient 3 Subscript 1 3 X 1= 3
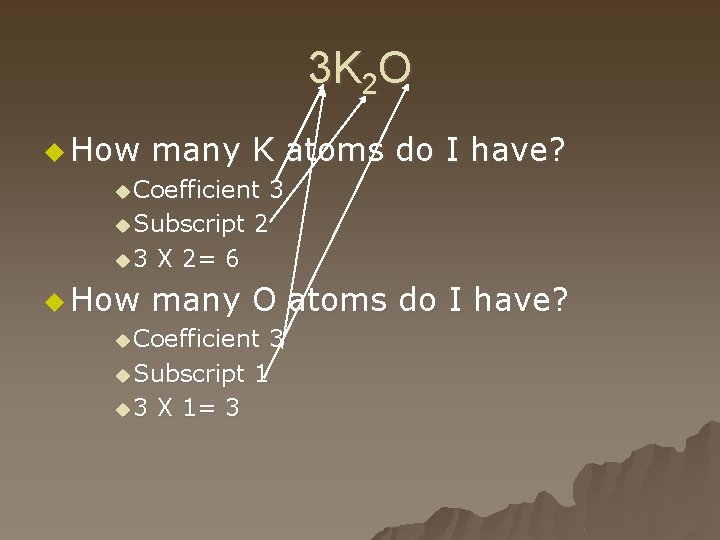
3 K 2 O u How many K atoms do I have? u Coefficient 3 u Subscript 2 u 3 X 2= 6 u How many O atoms do I have? u Coefficient 3 u Subscript 1 u 3 X 1= 3
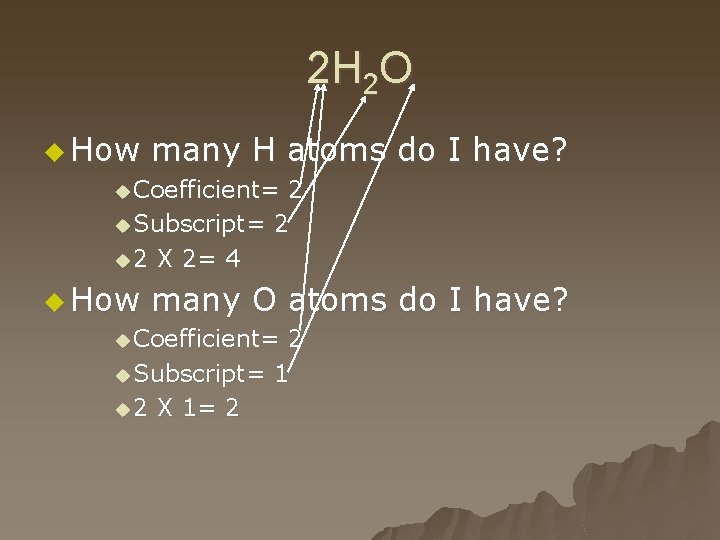
2 H 2 O u How many H atoms do I have? u Coefficient= 2 u Subscript= 2 u 2 X 2= 4 u How many O atoms do I have? u Coefficient= 2 u Subscript= 1 u 2 X 1= 2

K 3 PO 4 u How many K atoms do I have? u Coefficient= 1 u Subscript= 3 u 1 X 3= 3 u How many P atoms do I have? u Coefficient= 1 u Subscript= 1 u 1 X 1= 1 u How many O atoms do I have? u Coefficient= 1 u Subscript= 4 u 1 X 4= 4

Mg 3(PO 4)2 u How many Mg atoms do I have? u Coefficient= 1 u Subscript= 3 u 1 X 3= 3 u How many P atoms do I have? u Coefficient= 1 u Subscript=1 X 2= 2 u How many O atoms do I have? u Coefficient= 1 u Subscript= 4 X 2=8 u 1 X 8= 8
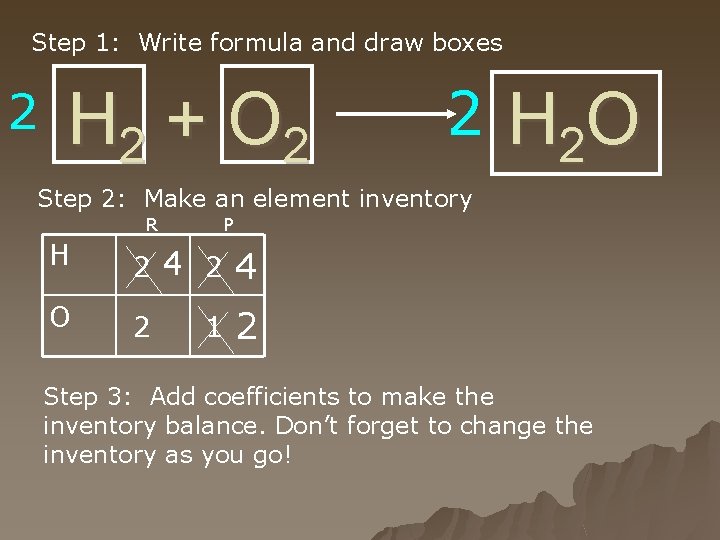
Step 1: Write formula and draw boxes 2 H 2 + O 2 2 H 2 O Step 2: Make an element inventory R P H 2 4 O 2 2 1 Step 3: Add coefficients to make the inventory balance. Don’t forget to change the inventory as you go!
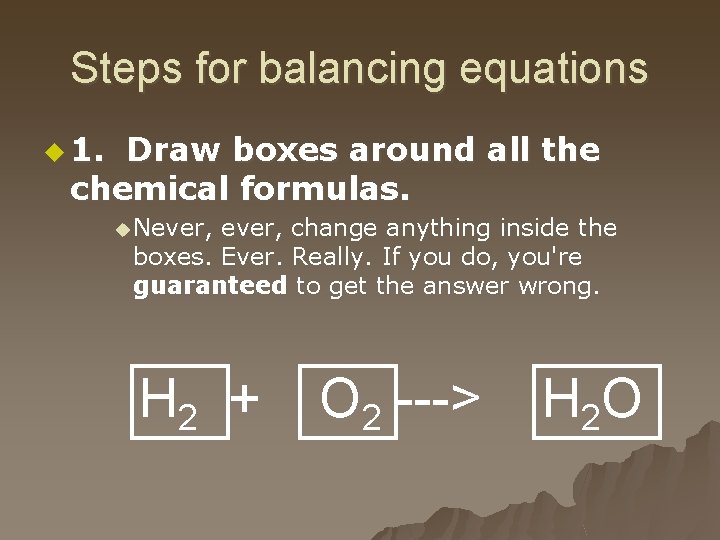
Steps for balancing equations u 1. Draw boxes around all the chemical formulas. u Never, change anything inside the boxes. Ever. Really. If you do, you're guaranteed to get the answer wrong. H 2 + O 2 ---> H 2 O

Steps for balancing equations u 2. Make an element inventory. u How are you going to know if the equation is balanced if you don't actually make a list of how many of each atom you have? You won't. You have to make an inventory of how many atoms of each element you have, and then you have to keep it current throughout the whole problem. u See example on next slide
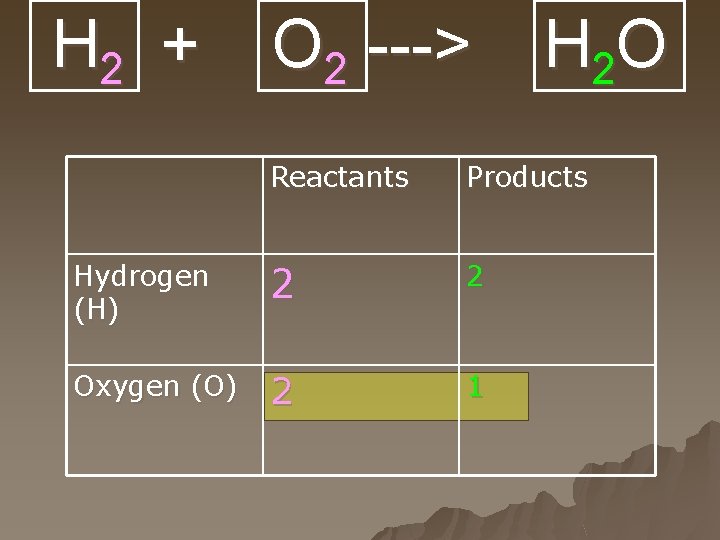
H 2 + O 2 ---> H 2 O Reactants Products Hydrogen (H) 2 2 Oxygen (O) 2 1

Steps for balancing equations u 3. Write numbers in front of each of the boxes until the inventory for each element is the same both before and after the reaction. u Whenever you change a number, make sure to update the inventory - otherwise, you run the risk of balancing it incorrectly. When all the numbers in the inventory balance, then the equation can balance,
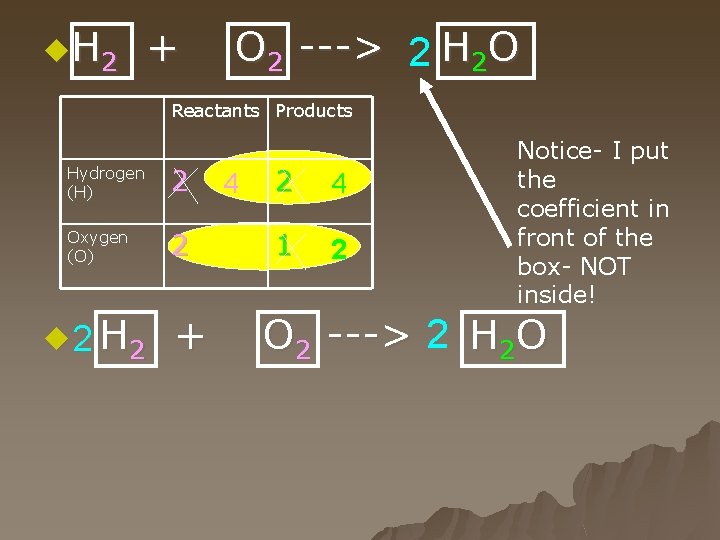
u. H 2 + O 2 ---> 2 H 2 O Reactants Products Hydrogen (H) 2 Oxygen (O) 2 u 2 H 2 + 4 2 4 1 2 Notice- I put the coefficient in front of the box- NOT inside! O 2 ---> 2 H 2 O

Steps for balancing equations u 4. Find the elements which appear in the fewest numbers of molecules and balance these first. u Continue in sequence until you balance the element which appears in the most molecules last. u Tip: Start by balancing an element that appears in only one reactant and product.

Rules You cannot change a subscript. u You cannot place a coefficient in the middle of a formula. H 2 + O 2 ---> H 22 O u Make sure that your final set of coefficients are all whole numbers with no common factors other than one. u For example, this equation is balanced: u u 4 H 2 + 2 O 2 ---> 4 H 2 O u However, all the coefficients have the common factor of two. Divide through to eliminate common factors like this.
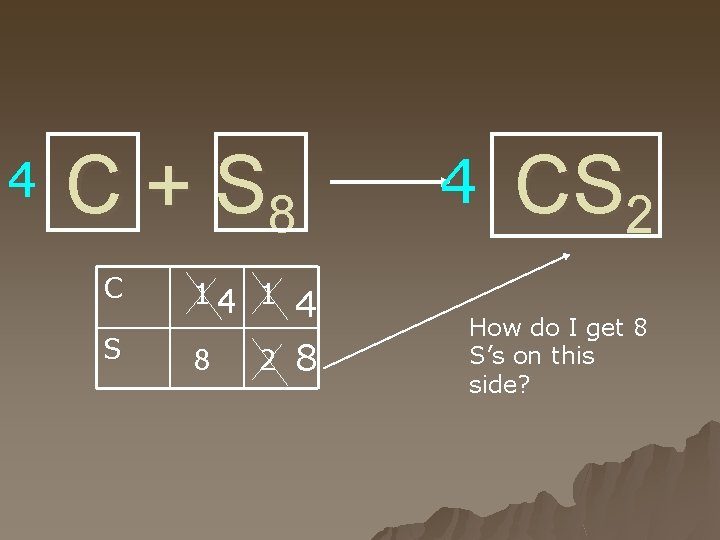
4 C + S 8 C S 14 1 8 2 4 8 4 CS 2 How do I get 8 S’s on this side?

If you run into problems trying to figure out the answer… u Find the lowest common denominator of those two numbers, and then put the numbers in front of those two boxes which allow the inventory on both sides to match.

If you run into problems trying to figure out the answer… What happens when the only way you can get a problem to work out is to make one of the numbers a decimal or fraction? u When this happens, find the largest molecule in the equation and stick a "2" in front of it. u Then start the problem over. u Will this work all the time? Well, no. But it will work sometimes, and give you a new strategy for hard problems. u
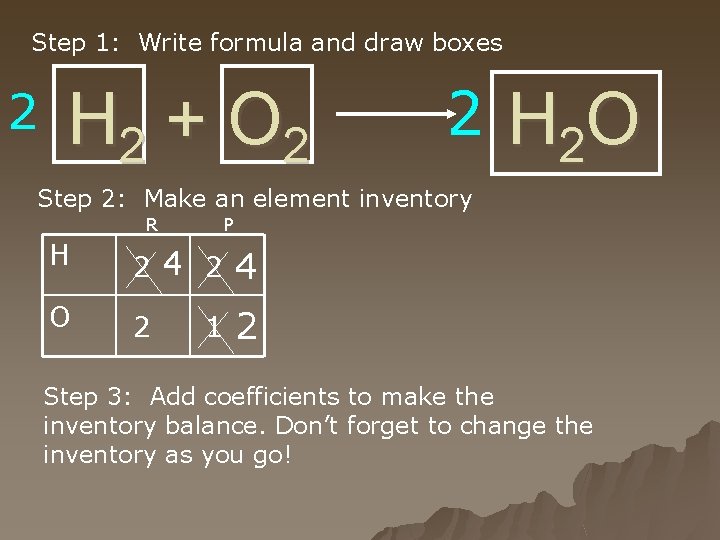
Step 1: Write formula and draw boxes 2 H 2 + O 2 2 H 2 O Step 2: Make an element inventory R P H 2 4 O 2 2 1 Step 3: Add coefficients to make the inventory balance. Don’t forget to change the inventory as you go!
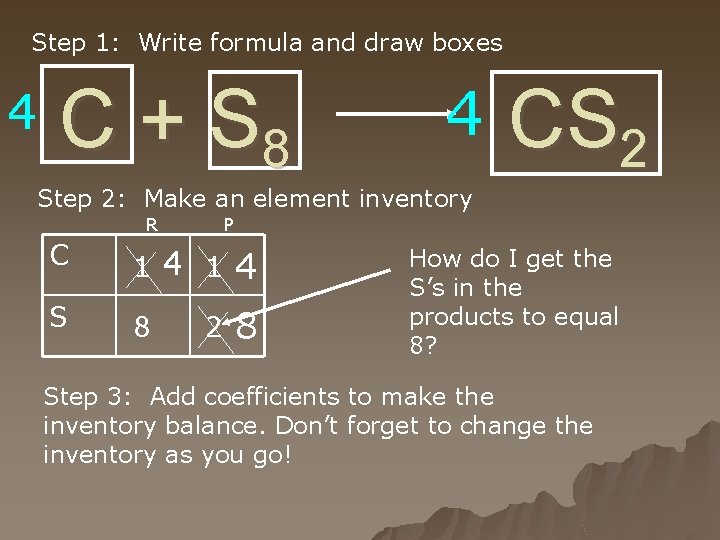
Step 1: Write formula and draw boxes 4 C + S 8 4 CS 2 Step 2: Make an element inventory R P C 1 4 S 8 8 2 How do I get the S’s in the products to equal 8? Step 3: Add coefficients to make the inventory balance. Don’t forget to change the inventory as you go!
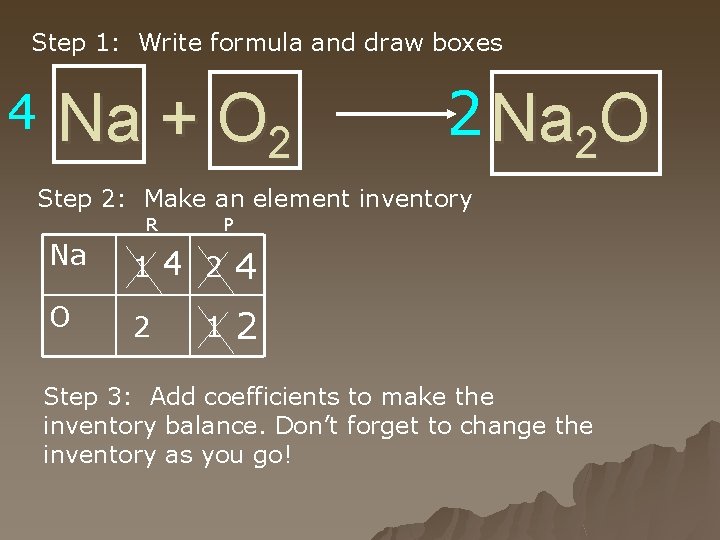
Step 1: Write formula and draw boxes 4 Na + O 2 2 Na 2 O Step 2: Make an element inventory R P Na 1 4 2 4 O 2 2 1 Step 3: Add coefficients to make the inventory balance. Don’t forget to change the inventory as you go!
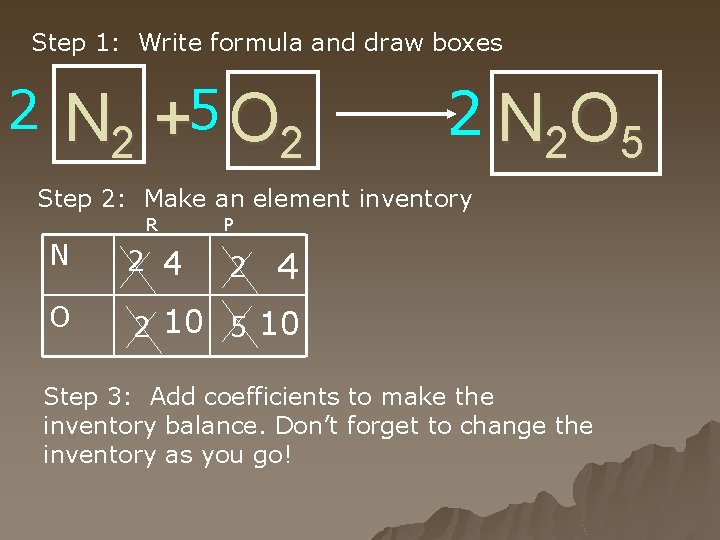
Step 1: Write formula and draw boxes 2 N 2 +5 O 2 2 N 2 O 5 Step 2: Make an element inventory R P N 2 4 O 2 10 5 10 2 4 Step 3: Add coefficients to make the inventory balance. Don’t forget to change the inventory as you go!
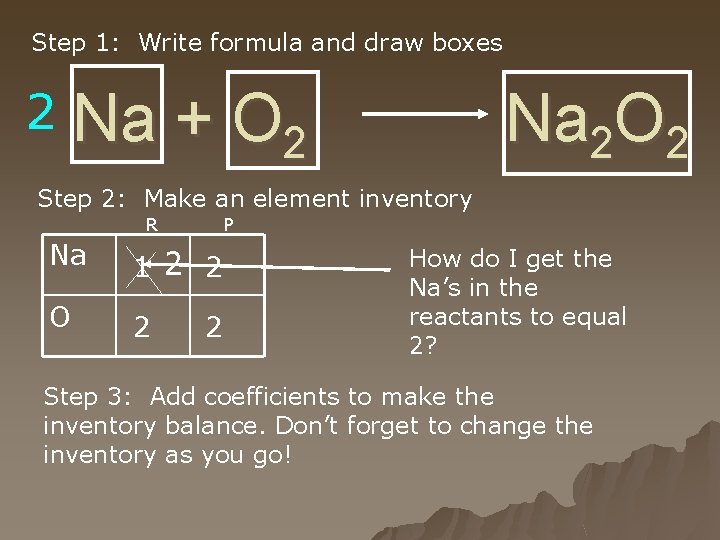
Step 1: Write formula and draw boxes 2 Na + O 2 Na 2 O 2 Step 2: Make an element inventory R P Na 1 2 2 O 2 2 How do I get the Na’s in the reactants to equal 2? Step 3: Add coefficients to make the inventory balance. Don’t forget to change the inventory as you go!
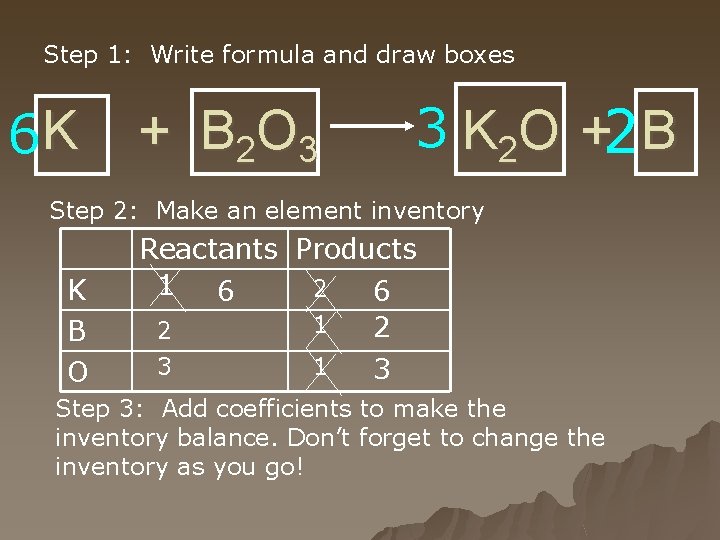
Step 1: Write formula and draw boxes 6 K + B 2 O 3 3 K 2 O +2 B Step 2: Make an element inventory K B O Reactants Products 1 2 6 6 1 2 2 3 1 3 Step 3: Add coefficients to make the inventory balance. Don’t forget to change the inventory as you go!
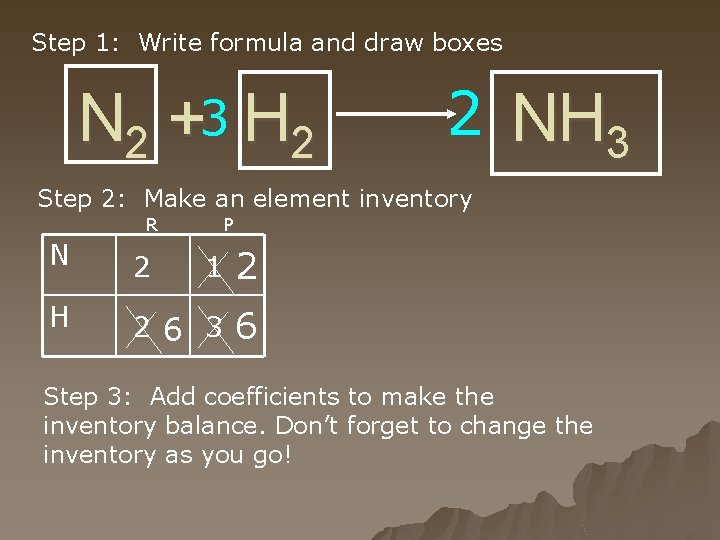
Step 1: Write formula and draw boxes N 2 +3 H 2 2 NH 3 Step 2: Make an element inventory R P N 2 1 2 H 2 6 3 6 Step 3: Add coefficients to make the inventory balance. Don’t forget to change the inventory as you go!
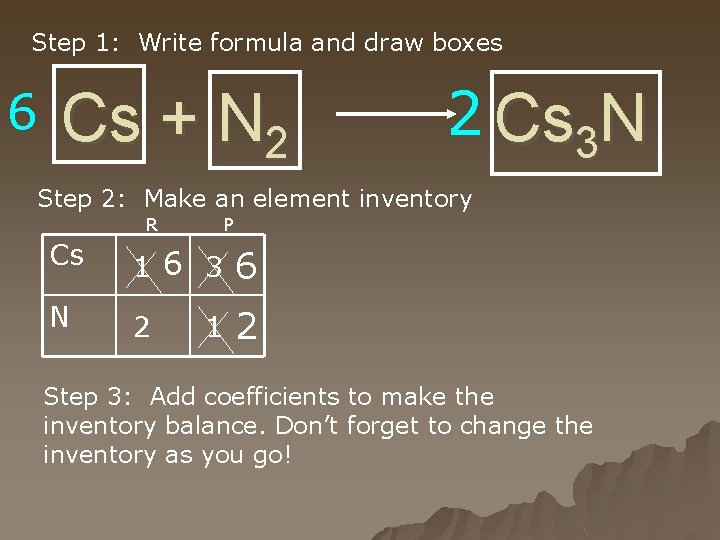
Step 1: Write formula and draw boxes 6 Cs + N 2 2 Cs 3 N Step 2: Make an element inventory R P Cs 1 6 3 6 N 2 2 1 Step 3: Add coefficients to make the inventory balance. Don’t forget to change the inventory as you go!
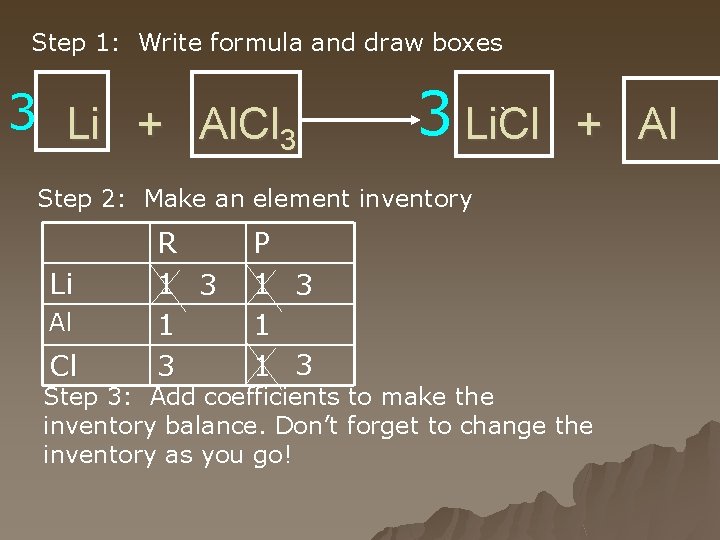
Step 1: Write formula and draw boxes 3 Li + Al. Cl 3 3 Li. Cl ` + Al Step 2: Make an element inventory Li Al Cl R 1 3 P 1 3 1 1 3 Step 3: Add coefficients to make the inventory balance. Don’t forget to change the inventory as you go!
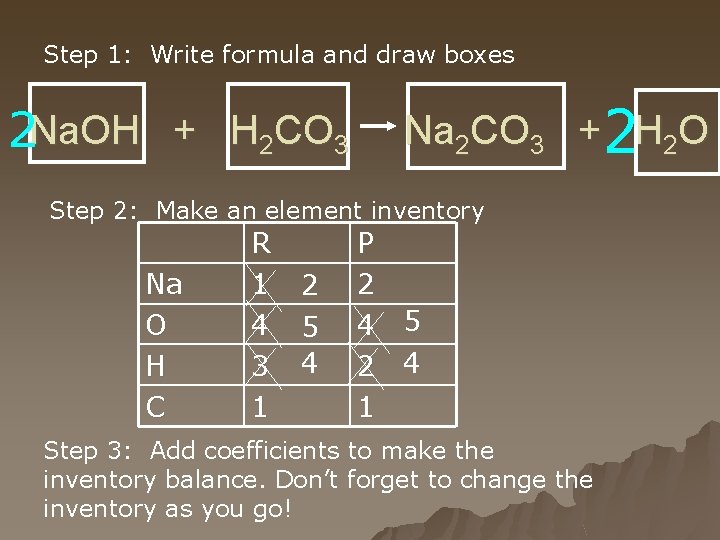
Step 1: Write formula and draw boxes 2 Na. OH + H 2 CO 3 Na 2 CO 3 +2 H 2 O Step 2: Make an element inventory Na O H C R 1 4 3 1 2 5 4 P 2 4 5 2 4 1 Step 3: Add coefficients to make the inventory balance. Don’t forget to change the inventory as you go!

Step 1: Write formula and draw boxes 4 CO 2 +6 H 2 O 2 C 2 H 6 +7 O 2 Step 2: Make an element inventory C H O R 2 4 6 12 2 14 P 1 2 3 4 12 8 14 Step 3: Add coefficients to make the inventory balance. Don’t forget to change the inventory as you go!
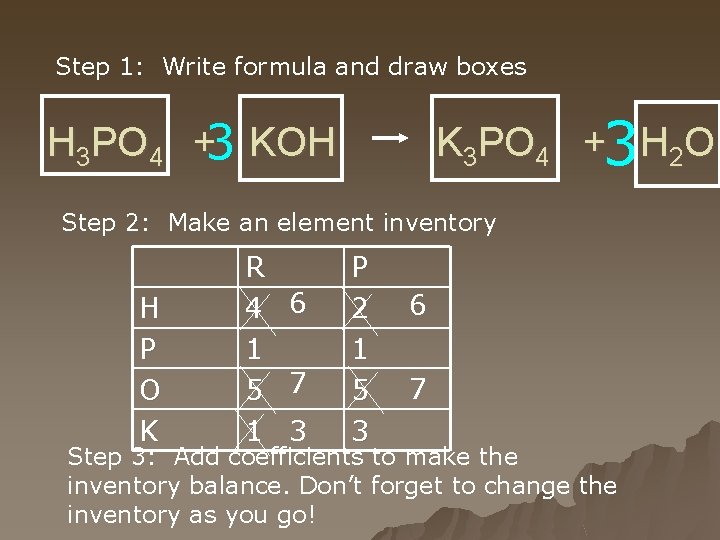
Step 1: Write formula and draw boxes K 3 PO 4 +3 H 2 O H 3 PO 4 +3 KOH Step 2: Make an element inventory H P O K R 4 6 1 5 7 1 3 P 2 1 5 3 6 7 Step 3: Add coefficients to make the inventory balance. Don’t forget to change the inventory as you go!

Step 1: Write formula and draw boxes 10 Na +2 Na. NO 3 6 Na 2 O + N 2 Step 2: Make an element inventory Na N O R 2 3 12 1 2 3 6 P 2 2 2 12 6 Step 3: Add coefficients to make the inventory balance. Don’t forget to change the inventory as you go!
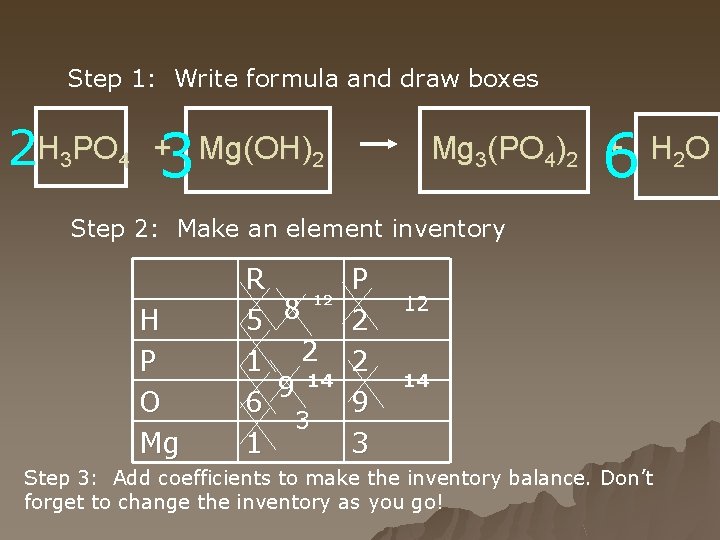
Step 1: Write formula and draw boxes 2 H 3 PO 4 3 6 + Mg(OH)2 Mg 3(PO 4)2 + H 2 O Step 2: Make an element inventory H P O Mg R 12 8 5 1 2 9 14 6 3 1 P 2 2 9 3 12 14 Step 3: Add coefficients to make the inventory balance. Don’t forget to change the inventory as you go!
- Slides: 40