Balancing Equations and Types of Equations Day 9

Balancing Equations and Types of Equations Day 9
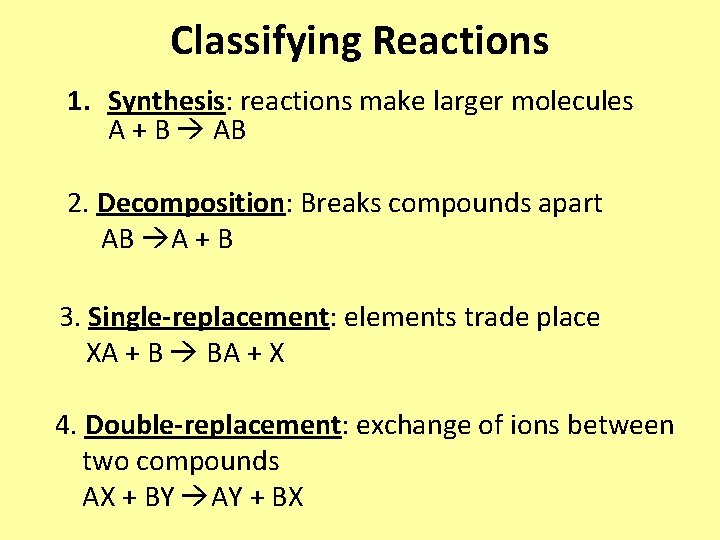
Classifying Reactions 1. Synthesis: reactions make larger molecules A + B AB 2. Decomposition: Breaks compounds apart AB A + B 3. Single-replacement: elements trade place XA + B BA + X 4. Double-replacement: exchange of ions between two compounds AX + BY AY + BX

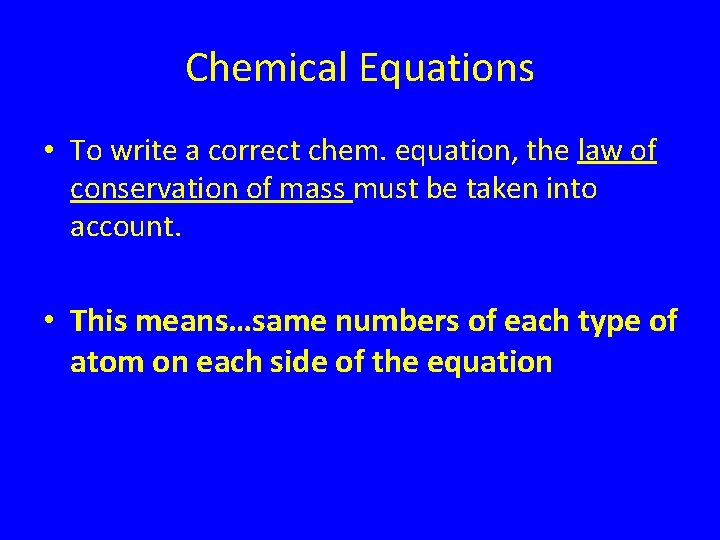
Chemical Equations • To write a correct chem. equation, the law of conservation of mass must be taken into account. • This means…same numbers of each type of atom on each side of the equation

Balancing Chem. Equations • Balancing of chem. equations is done by adding coefficients (number in front of the element or compound) Al 2 Al + S + 3 S Al 2 S 3 Not Balanced

Never…. • Never change a subscript to balance an equation. – If you change the formula you are describing a different reaction. – H 2 O is a different compound than H 2 O 2 Never put a coefficient in the middle of a formula – 2 Na. Cl is okay, Na 2 Cl is not.
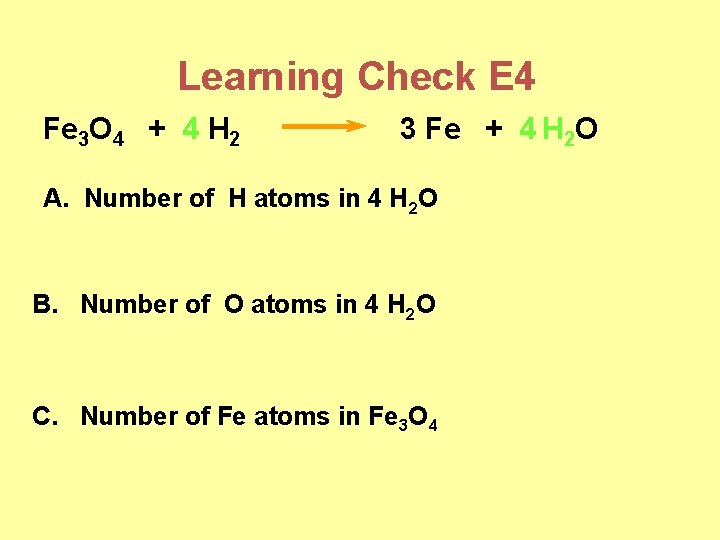
Learning Check E 4 Fe 3 O 4 + 4 H 2 3 Fe + 4 H 2 O A. Number of H atoms in 4 H 2 O B. Number of O atoms in 4 H 2 O C. Number of Fe atoms in Fe 3 O 4
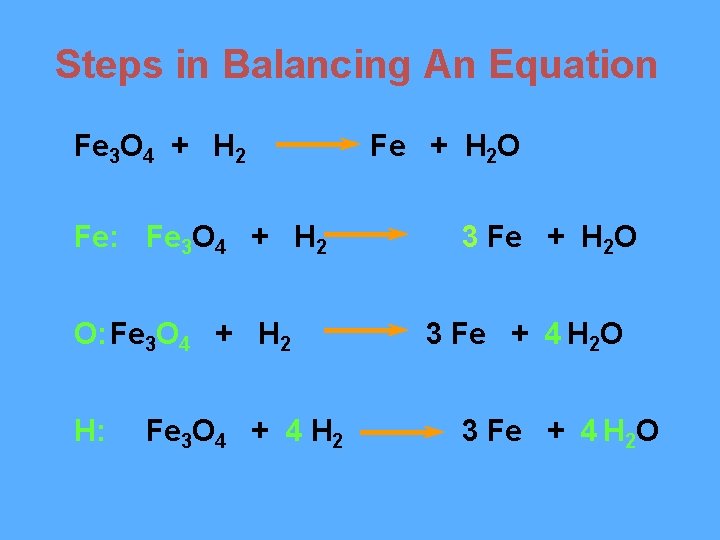
Steps in Balancing An Equation Fe 3 O 4 + H 2 Fe: Fe 3 O 4 + H 2 O: Fe 3 O 4 + H 2 H: Fe 3 O 4 + 4 H 2 Fe + H 2 O 3 Fe + 4 H 2 O
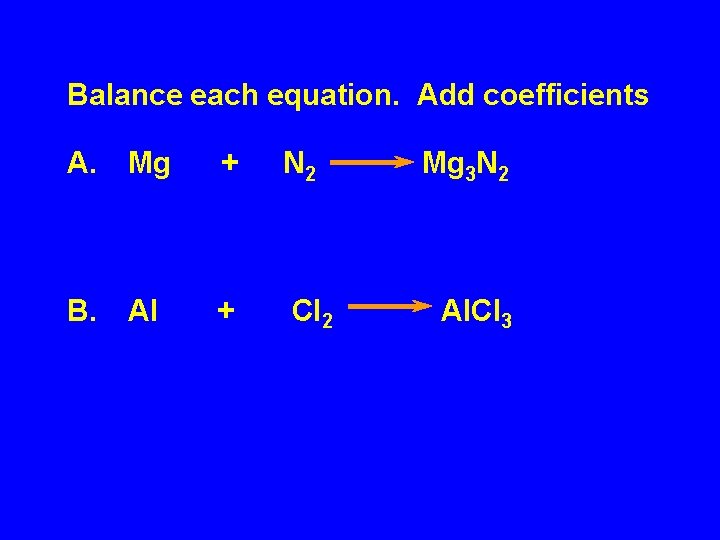
Balance each equation. Add coefficients A. Mg + N 2 B. Al + Cl 2 Mg 3 N 2 Al. Cl 3
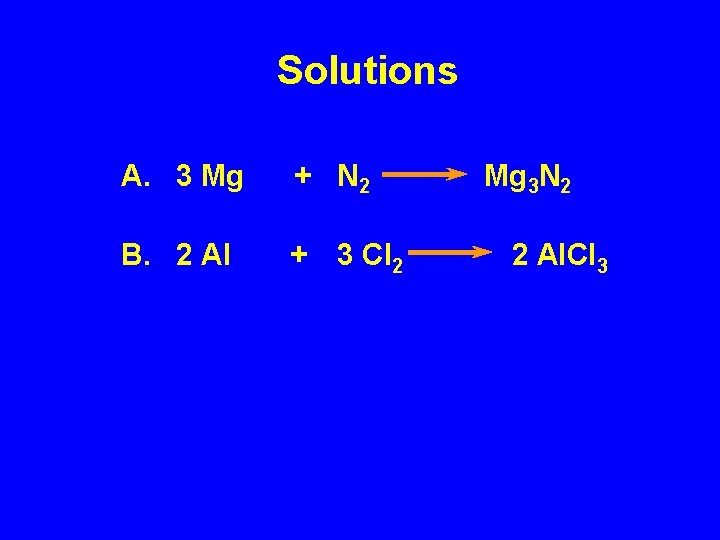
Solutions A. 3 Mg + N 2 B. 2 Al + 3 Cl 2 Mg 3 N 2 2 Al. Cl 3
- Slides: 10