Balancing Chemical Reactions Law of conservation of matter

Balancing Chemical Reactions

Law of conservation of matter �Chemical reactions must obey the law of conservation of matter. Law of conservation of matter states that matter is neither created nor destroyed, it only changes forms (becomes rearranged)

Law of conservation of matter �So the number of atoms on the reactant side must equal the number of atoms on the product side Reactants = products Reactants products

�Paperclip lab
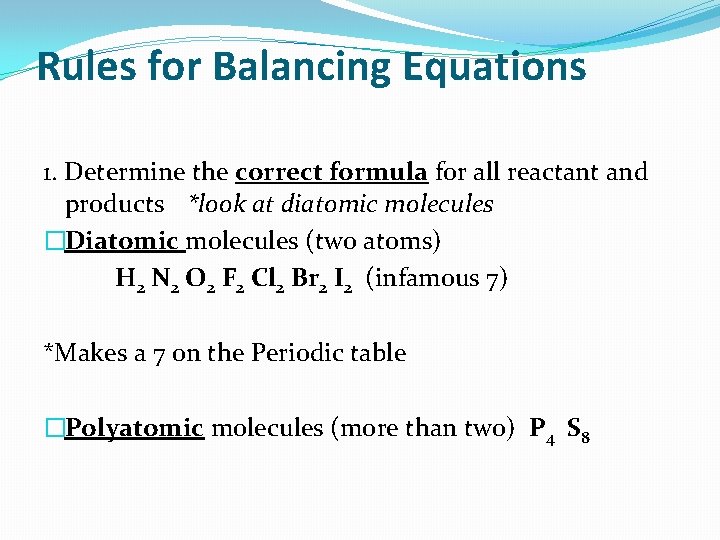
Rules for Balancing Equations 1. Determine the correct formula for all reactant and products *look at diatomic molecules �Diatomic molecules (two atoms) H 2 N 2 O 2 F 2 Cl 2 Br 2 I 2 (infamous 7) *Makes a 7 on the Periodic table �Polyatomic molecules (more than two) P 4 S 8
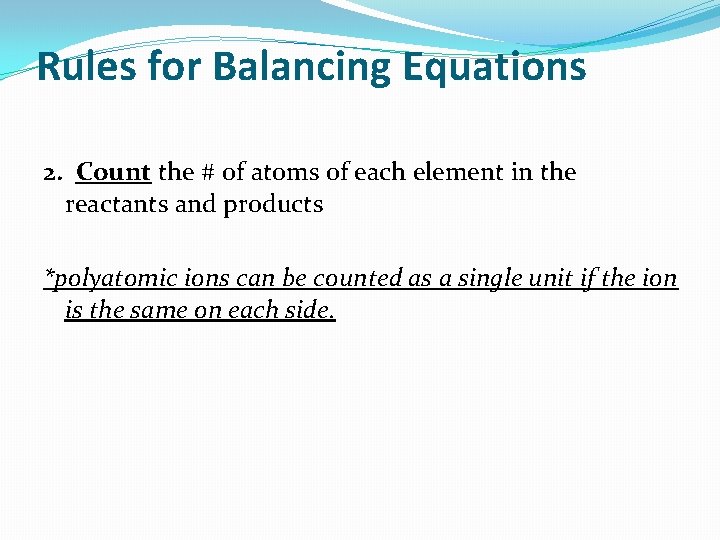
Rules for Balancing Equations 2. Count the # of atoms of each element in the reactants and products *polyatomic ions can be counted as a single unit if the ion is the same on each side.
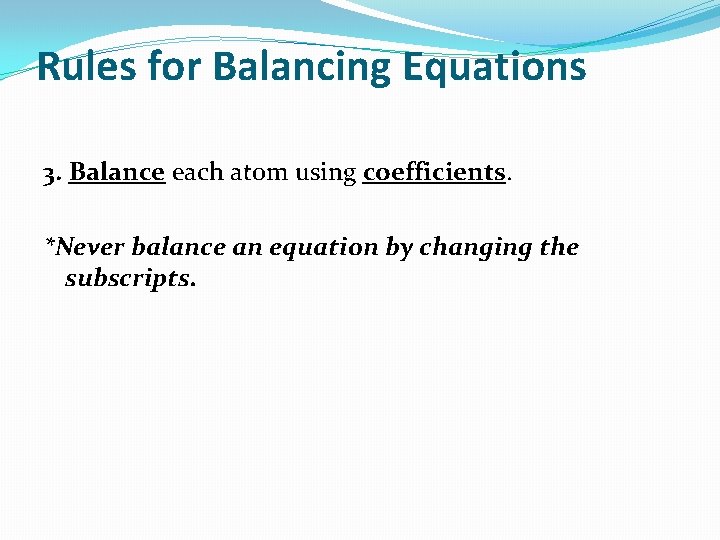
Rules for Balancing Equations 3. Balance each atom using coefficients. *Never balance an equation by changing the subscripts.

Rules for Balancing Equations 4. Check each atom or polyatomic ion to be sure that the equation is balanced.

Helpful hints: 1. All elements have a charge of zero when uncombined. (no ionic charge) 2. Leave H and O until the end 3. If stuck, go back to the beginning and put a 2 in front of the most complex chemical

Examples Boron reacts with lead(II) hydroxide to produce boron hydroxide and lead. Sulfur plus oxygen yield sulfur dioxide Sodium sulfate and calcium borate react to make sodium borate and calcium sulfate. Diphosphorus trioxide combines with water to create phosphorous acid.
- Slides: 10