Balancing Chemical Equations We will learn to balance
Balancing Chemical Equations We will learn to balance chemical equations. I will use a model to balance a set of chemical equations. Success Criteria: Know how the law of conservation of mass applies to balancing equations. Know the difference between subscripts and coefficients. Be able to balance equations.
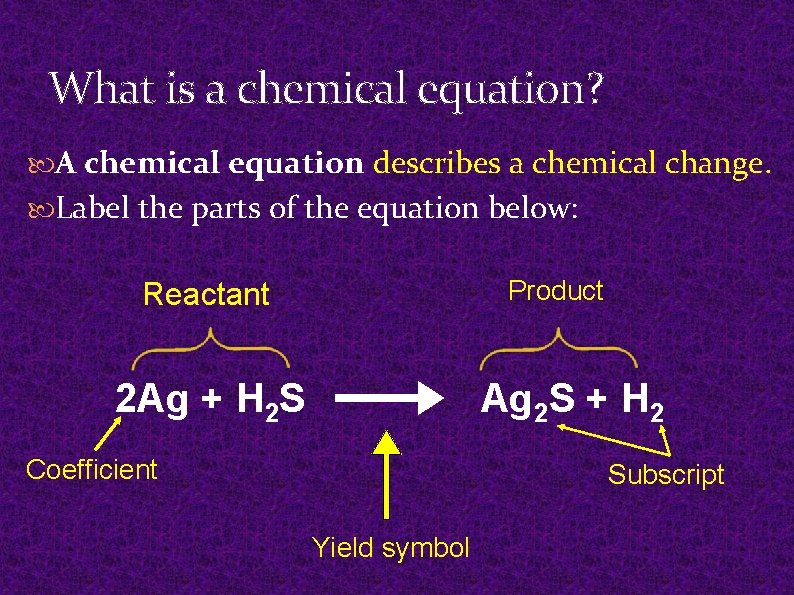
What is a chemical equation? A chemical equation describes a chemical change. Label the parts of the equation below: Product Reactant 2 Ag + H 2 S Ag 2 S + H 2 Coefficient Subscript Yield symbol

Reactants and Products Yield symbol – indicates direction of reaction Reactant - The chemicals that you react together. Written on the left side of equation. Products - The new chemicals formed by the reaction. Written on the right side of equation.
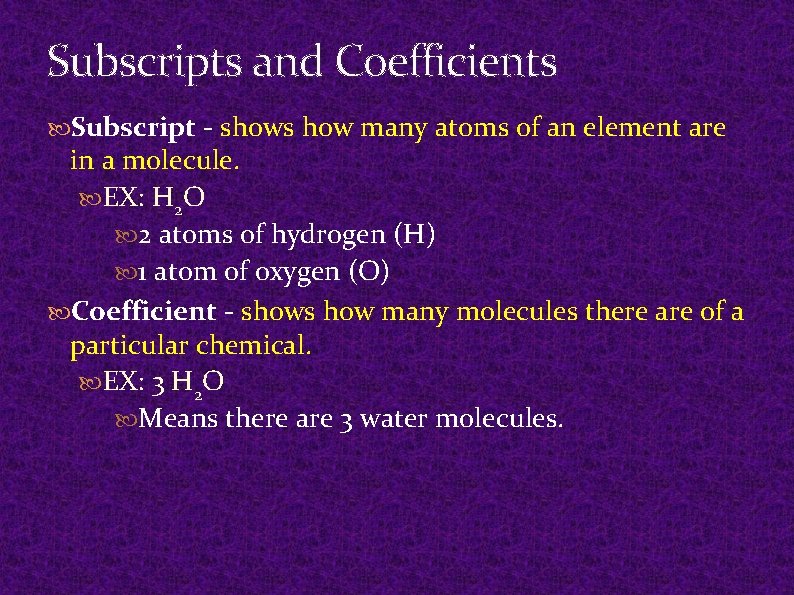
Subscripts and Coefficients Subscript - shows how many atoms of an element are in a molecule. EX: H 2 O 2 atoms of hydrogen (H) 1 atom of oxygen (O) Coefficient - shows how many molecules there are of a particular chemical. EX: 3 H 2 O Means there are 3 water molecules.

A Chemical Reaction 2 H 2 + O 2 2 H 2 O

Law of Conservation of Mass In a chemical reaction, matter is neither created nor destroyed. A balanced equation obeys the Law of Conservation of Mass. The number and type of atoms going INTO a reaction must be the same as the number and type of atoms coming OUT.
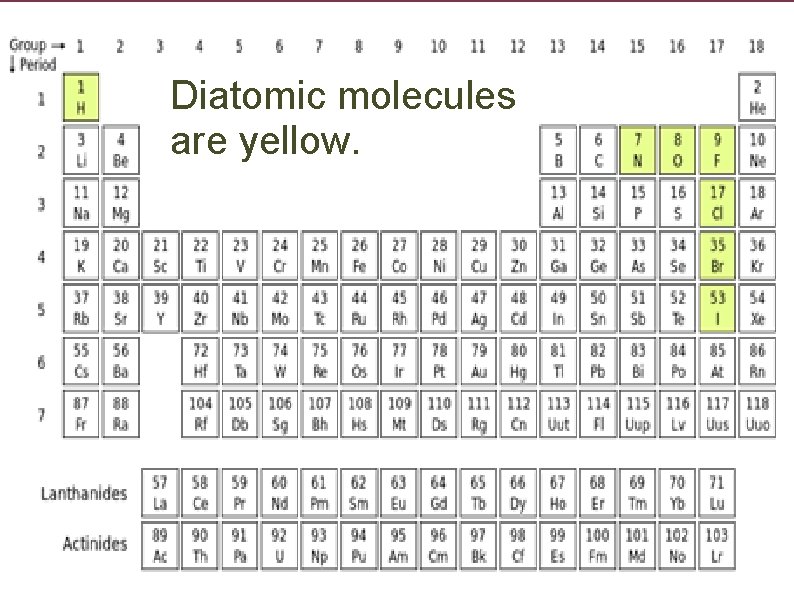
Diatomic molecules are yellow.
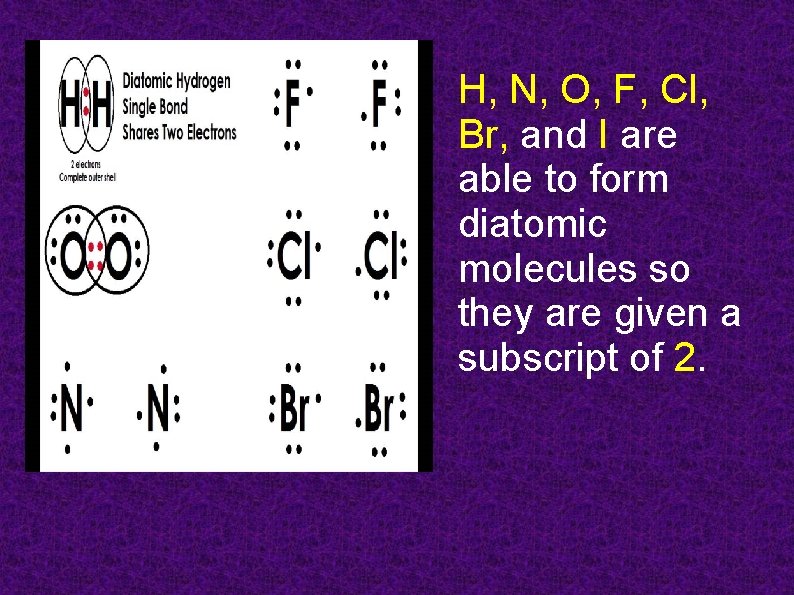
H, N, O, F, Cl, Br, and I are able to form diatomic molecules so they are given a subscript of 2.
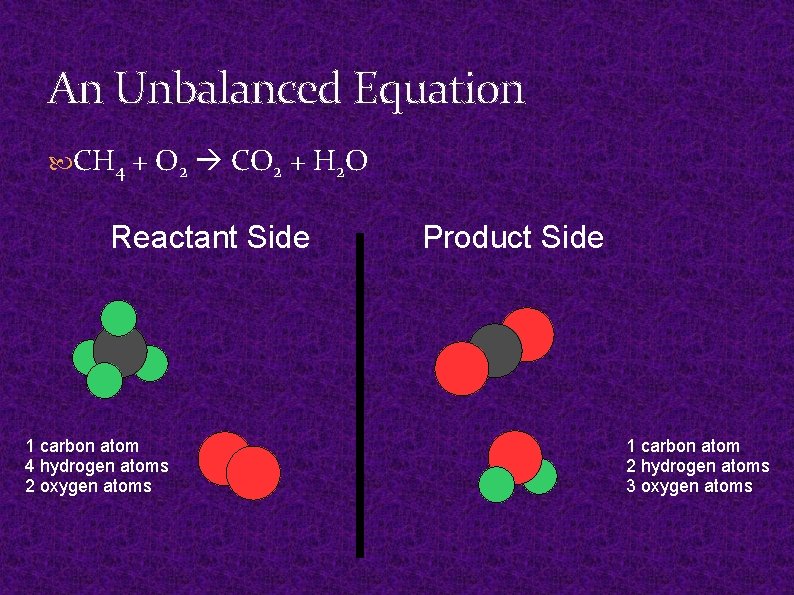
An Unbalanced Equation CH 4 + O 2 CO 2 + H 2 O Reactant Side 1 carbon atom 4 hydrogen atoms 2 oxygen atoms Product Side 1 carbon atom 2 hydrogen atoms 3 oxygen atoms
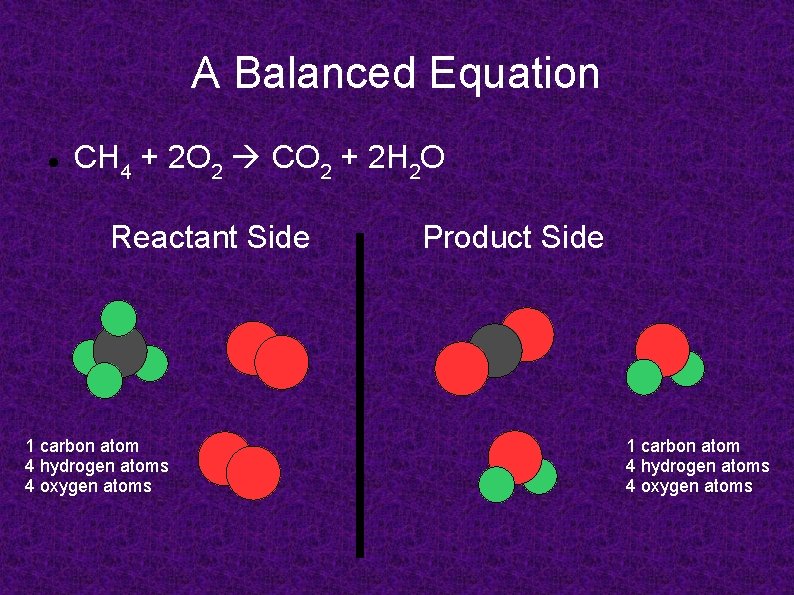
A Balanced Equation CH 4 + 2 O 2 CO 2 + 2 H 2 O Reactant Side 1 carbon atom 4 hydrogen atoms 4 oxygen atoms Product Side 1 carbon atom 4 hydrogen atoms 4 oxygen atoms

Rules of the Game 1. Matter cannot be created or destroyed. 2. Subscripts cannot be added, removed, or changed. 3. You can only change coefficients. 4. Coefficients can only go in front of chemical formulas. . . NEVER in the middle of a formula.

Rules of the Game – Extra Tips Try balancing big formulas first; save free elements for last. If the same polyatomic ion appears on both sides of the equation, it’s usually okay to treat it as one unit. There is no one particular way to balance equations. Some equations are harder to balance than others and might require some creativity to solve.
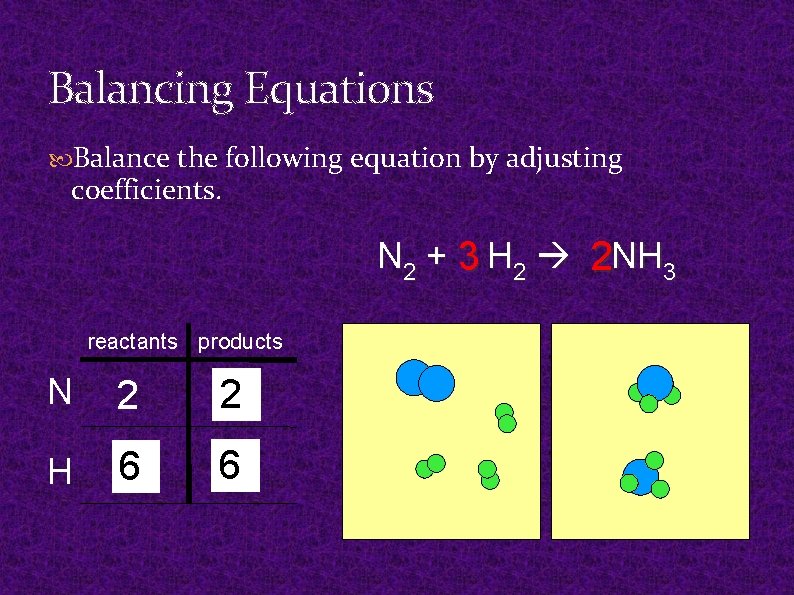
Balancing Equations Balance the following equation by adjusting coefficients. N 2 + 3 H 2 2 NH 3 reactants products N 2 21 H 6 2 63
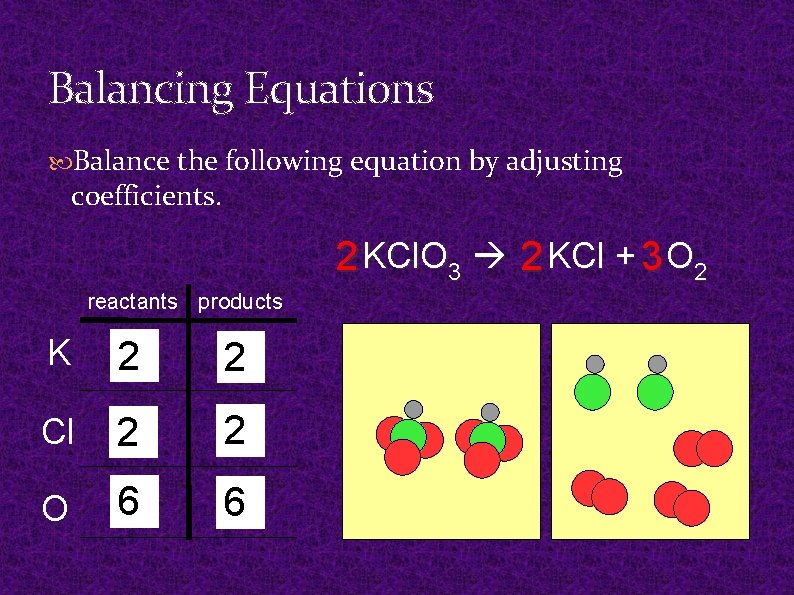
Balancing Equations Balance the following equation by adjusting coefficients. 2 KCl. O 3 2 KCl + 3 O 2 reactants products K 2 1 12 Cl 2 1 O 6 3 6 2
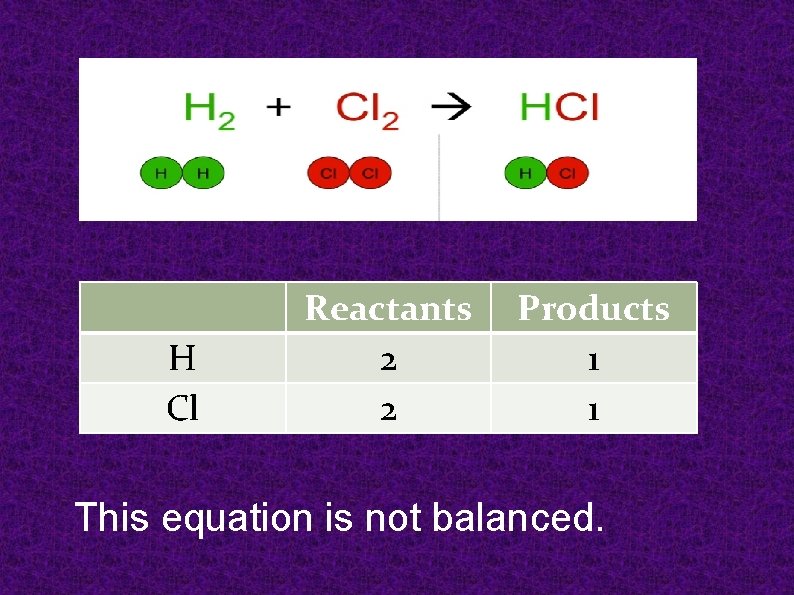
H Cl Reactants 2 2 Products 1 1 This equation is not balanced.
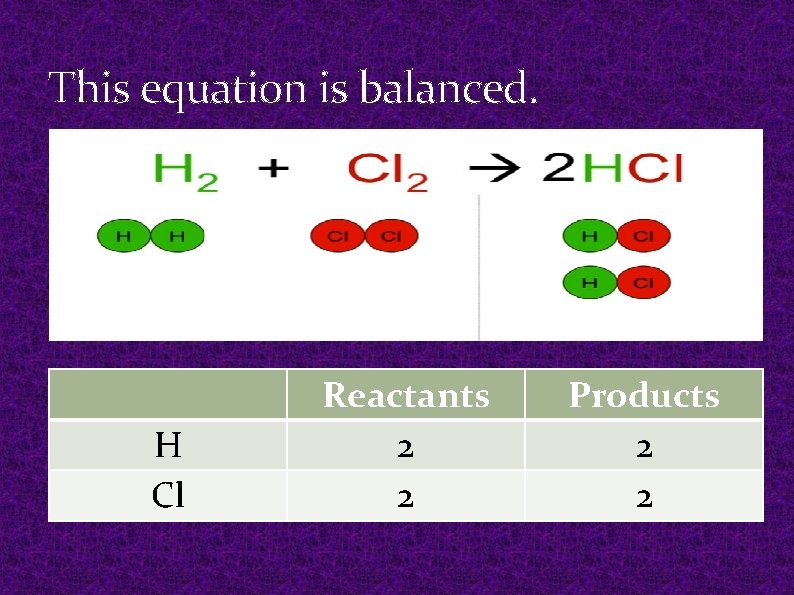
This equation is balanced. H Cl Reactants 2 2 Products 2 2

- Slides: 17