Balancing Chemical Equations Subscripts show ratio of atoms

Balancing Chemical Equations

Subscripts show ratio of atoms of elements in a compound e. g. CO 2 has one C atom to every two O atoms Practice: Fe 2 O 3 – how many Fe atoms? – how many O atoms? Subscripts cannot be changed!

Coefficients Gives number of molecules of a compound Coefficient multiplied by subscript gives numbers of atoms of each element e. g. 3 H 2 O means there are 3 molecules of H 2 O – How many atoms of H total are there? – How many atoms of O total are there? CAN change coefficient numbers

Conservation of Mass NO atoms are lost during reactions!! Start with 13 g Fe and 0. 5 g O, end with 13. 5 g of Fe 2 O 3. mass of reactants MUST equal mass of products SO we balance chemical equations so there are the same number of atoms on each side of an equation

Simple Problem: H 2 + O 2 = H 2 O – – In reactants: atoms of hydrogen? • 2 atoms of oxygen? In product: atoms of hydrogen? • 2 • 1 atoms of oxygen? PROBLEM – equation is not balanced.

Balancing H 2 + O 2 = H 2 O – – Change equation to H 2 O 2? NO!! Can’t change subscripts! But CAN change coefficients, so: H 2 + O 2 = 2 H 2 O 4 atoms of H and 2 of O STILL not balanced 2 H 2 + O 2 = 2 H 2 O four H atoms and 2 O atoms for reactants, and 4 H atoms and 2 O atoms for product Conservation of Mass is satisfied.
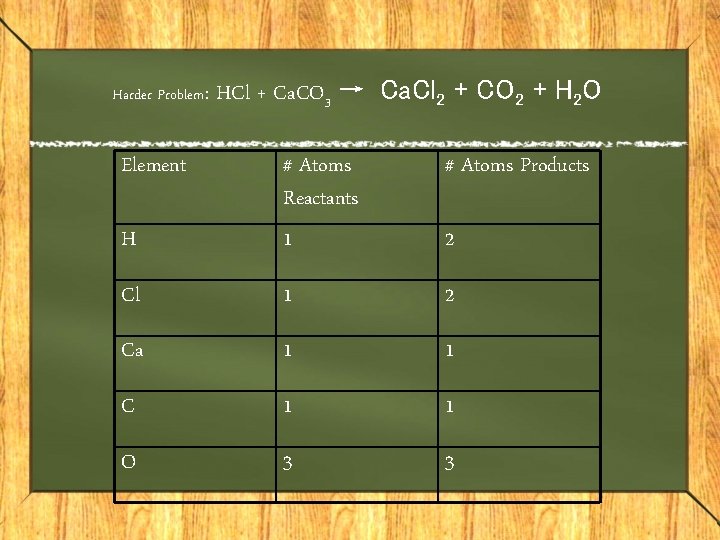
Harder Problem: Element HCl + Ca. CO 3 → Ca. Cl 2 + CO 2 + H 2 O # Atoms Products H # Atoms Reactants 1 Cl 1 2 Ca 1 1 C 1 1 O 3 3 2
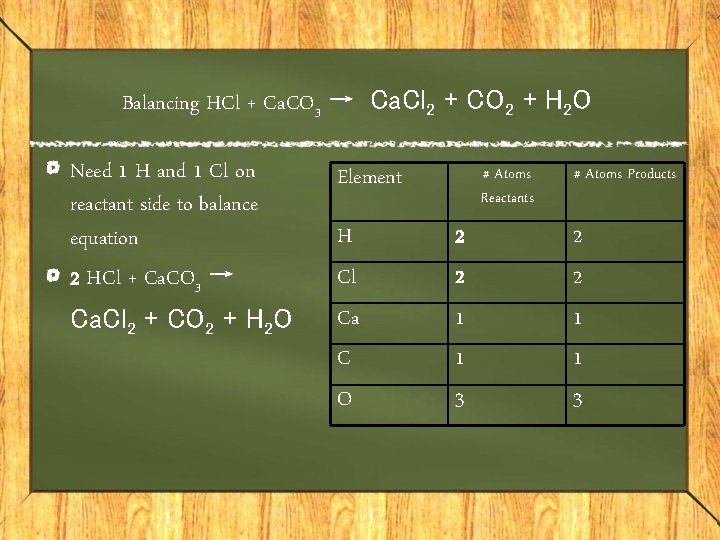
Balancing HCl + Ca. CO 3 → Ca. Cl 2 + CO 2 + H 2 O Need 1 H and 1 Cl on reactant side to balance equation 2 HCl + Ca. CO 3 → Ca. Cl 2 + CO 2 + H 2 O Element H Cl Ca C O # Atoms Reactants 2 2 1 1 3 # Atoms Products 2 2 1 1 3
- Slides: 8