Balancing Chemical Equations Ms Cook All chemical reactions

Balancing Chemical Equations Ms. Cook
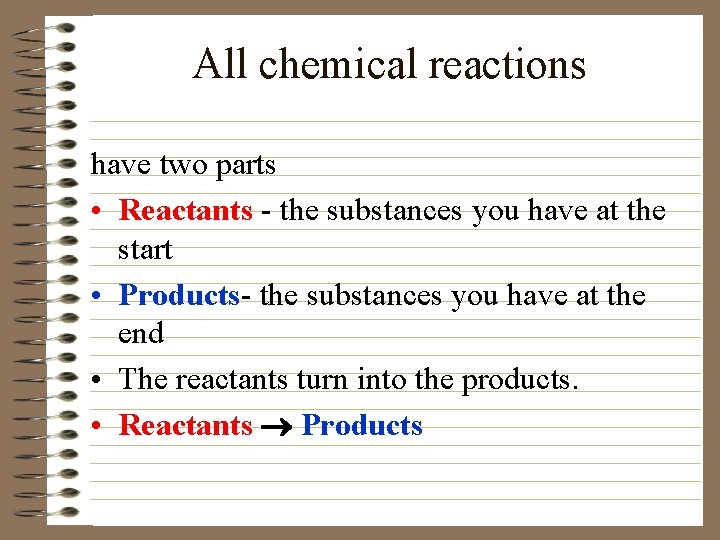
All chemical reactions have two parts • Reactants - the substances you have at the start • Products- the substances you have at the end • The reactants turn into the products. • Reactants ® Products
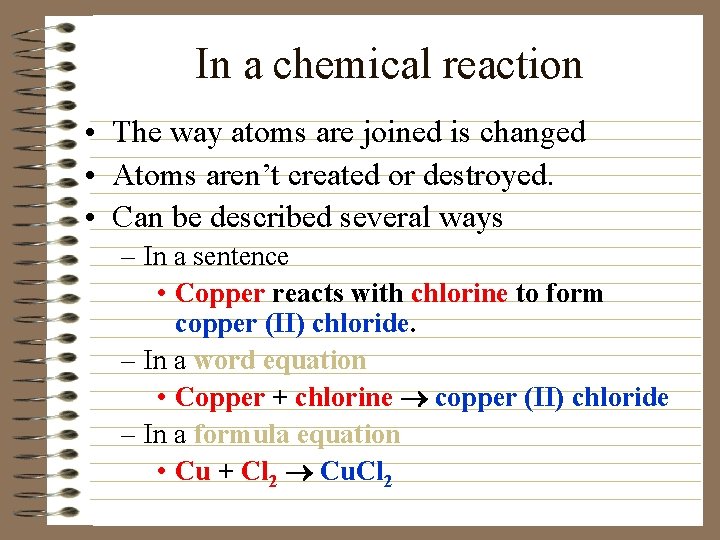
In a chemical reaction • The way atoms are joined is changed • Atoms aren’t created or destroyed. • Can be described several ways – In a sentence • Copper reacts with chlorine to form copper (II) chloride. – In a word equation • Copper + chlorine ® copper (II) chloride – In a formula equation • Cu + Cl 2 ® Cu. Cl 2
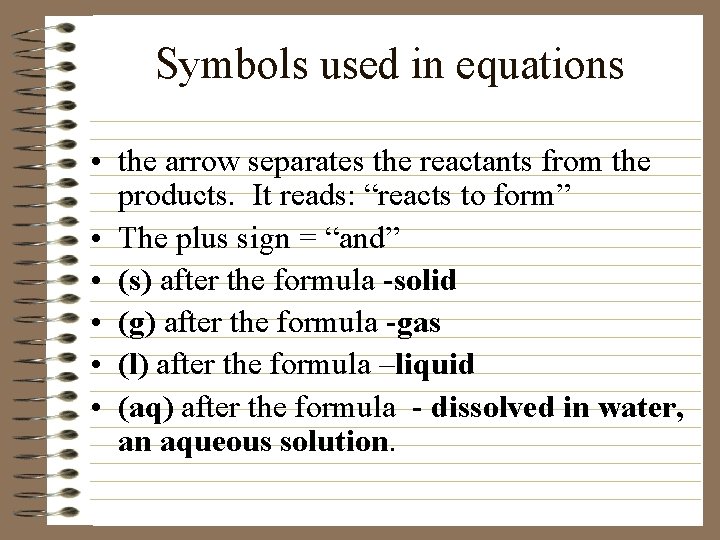
Symbols used in equations • the arrow separates the reactants from the products. It reads: “reacts to form” • The plus sign = “and” • (s) after the formula -solid • (g) after the formula -gas • (l) after the formula –liquid • (aq) after the formula - dissolved in water, an aqueous solution.
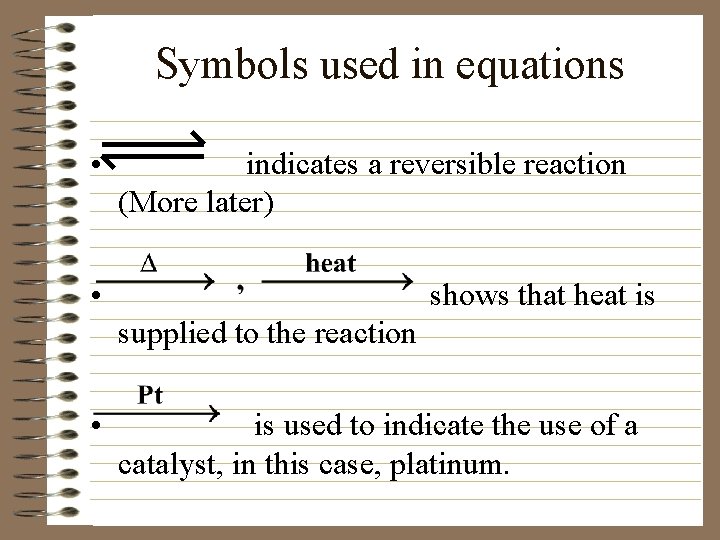
Symbols used in equations • indicates a reversible reaction (More later) • shows that heat is supplied to the reaction • is used to indicate the use of a catalyst, in this case, platinum.
What is a catalyst? • A substance that speeds up a reaction without being changed by the reaction. • Enzymes are biological or protein catalysts.
Skeleton Equation • Uses formulas and symbols to describe a reaction • doesn’t indicate how many.
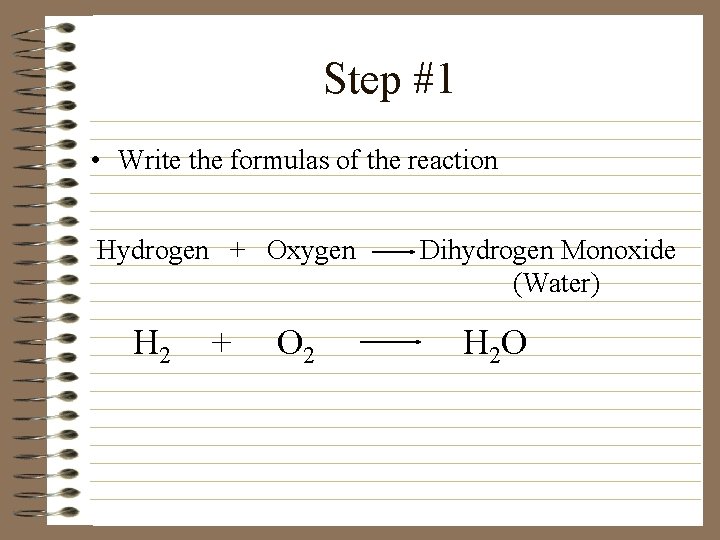
Step #1 • Write the formulas of the reaction Hydrogen + Oxygen H 2 + O 2 Dihydrogen Monoxide (Water) H 2 O
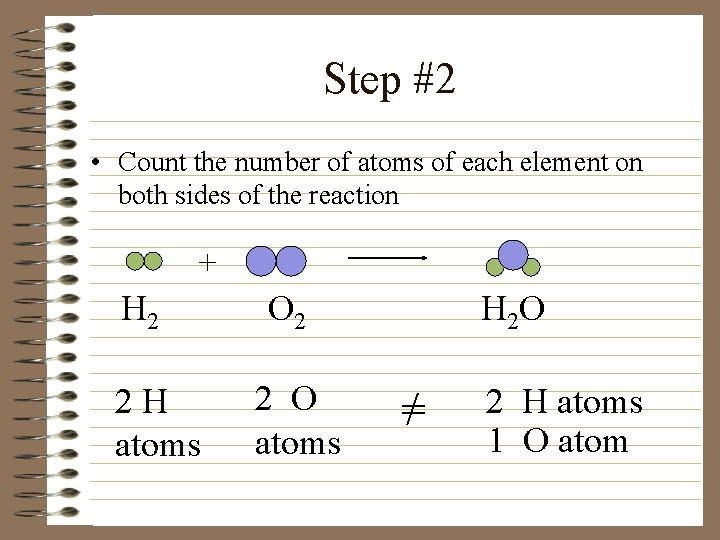
Step #2 • Count the number of atoms of each element on both sides of the reaction + H 2 2 H atoms O 2 2 O atoms H 2 O =/ 2 H atoms 1 O atom
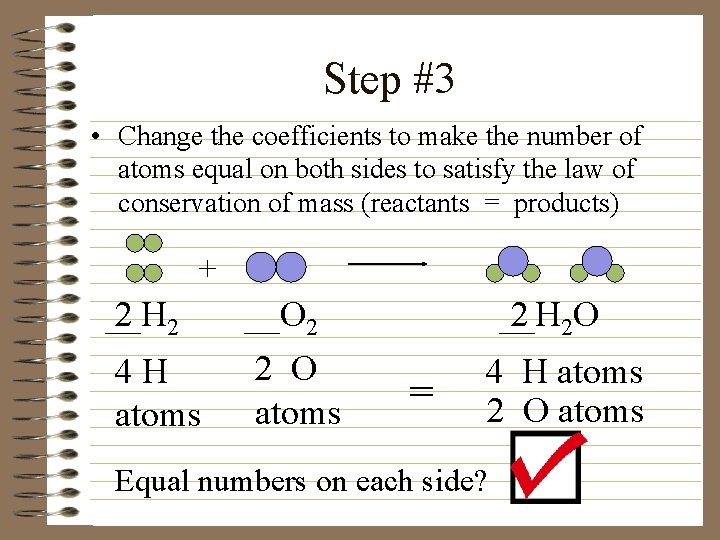
Step #3 • Change the coefficients to make the number of atoms equal on both sides to satisfy the law of conservation of mass (reactants = products) + __H 2 2 4 H atoms __O 2 2 O atoms __H 2 2 O = 4 H atoms 2 O atoms Equal numbers on each side?
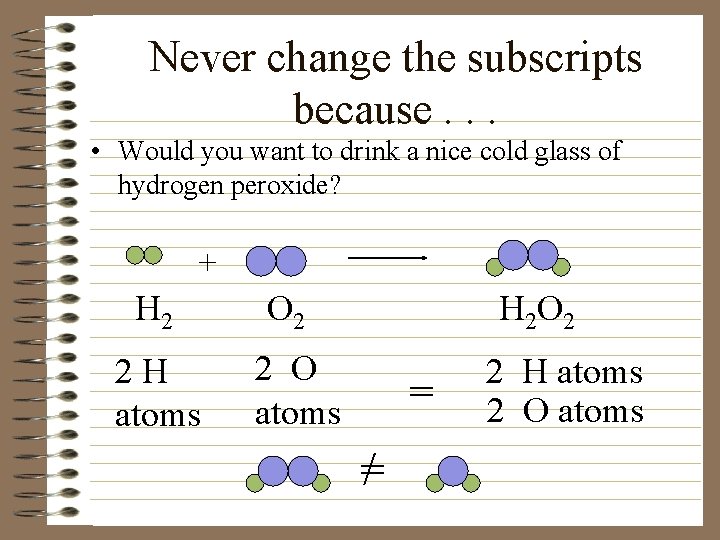
Never change the subscripts because. . . • Would you want to drink a nice cold glass of hydrogen peroxide? + H 2 2 H atoms O 2 H 2 O 2 2 O atoms = =/ 2 H atoms 2 O atoms
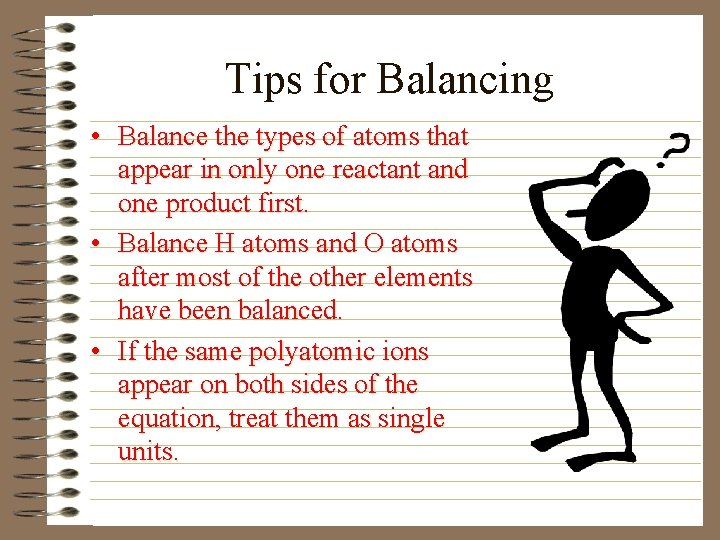
Tips for Balancing • Balance the types of atoms that appear in only one reactant and one product first. • Balance H atoms and O atoms after most of the other elements have been balanced. • If the same polyatomic ions appear on both sides of the equation, treat them as single units.
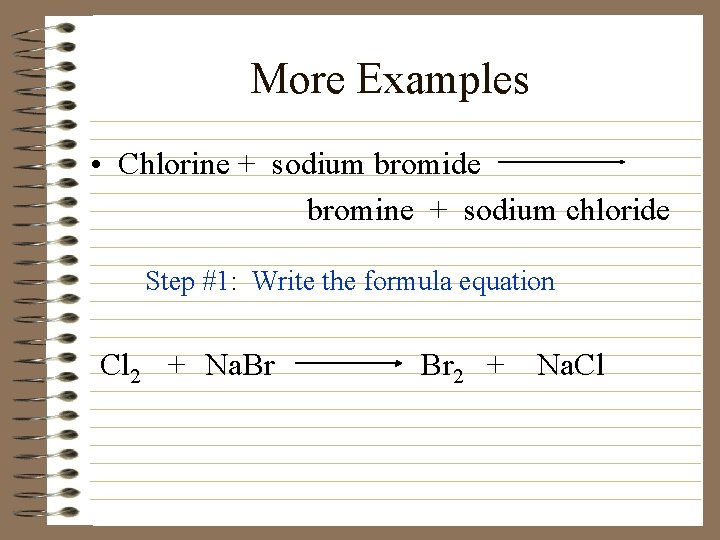
More Examples • Chlorine + sodium bromide bromine + sodium chloride Step #1: Write the formula equation Cl 2 + Na. Br Br 2 + Na. Cl
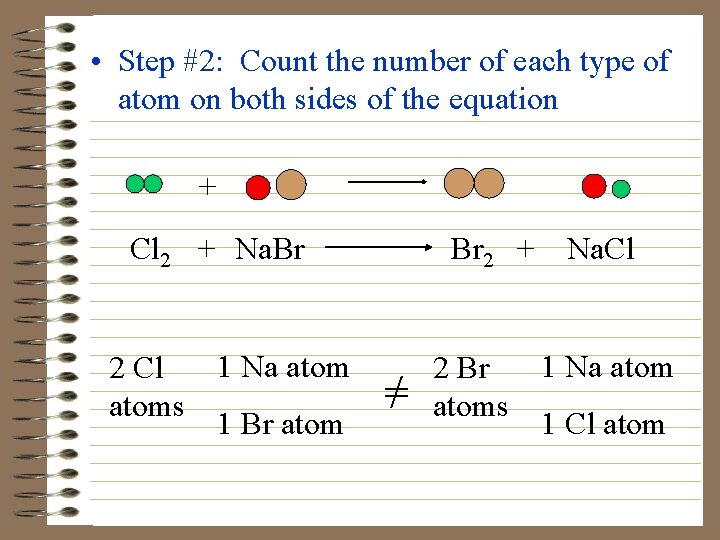
• Step #2: Count the number of each type of atom on both sides of the equation + Cl 2 + Na. Br 1 Na atom 2 Cl atoms 1 Br atom Br 2 + Na. Cl =/ 1 Na atom 2 Br atoms 1 Cl atom
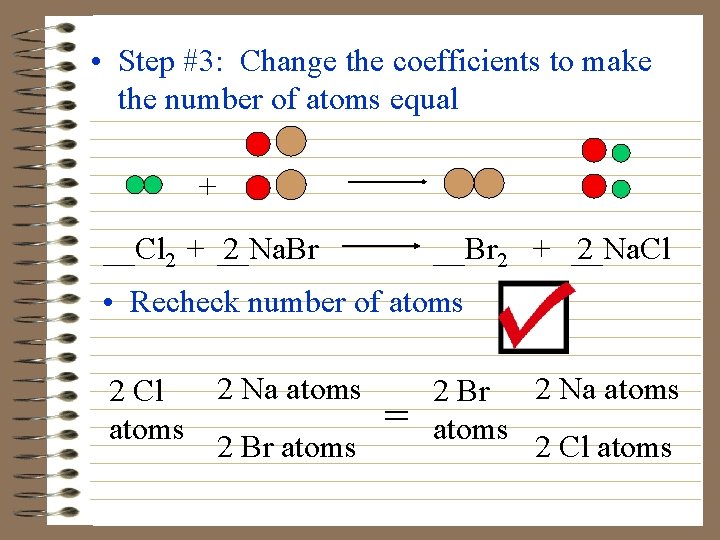
• Step #3: Change the coefficients to make the number of atoms equal + __Cl 2 + __Na. Br 2 __Br 2 + __Na. Cl 2 • Recheck number of atoms 2 Na atoms 2 Cl atoms 2 Br atoms = 2 Br 2 Na atoms 2 Cl atoms
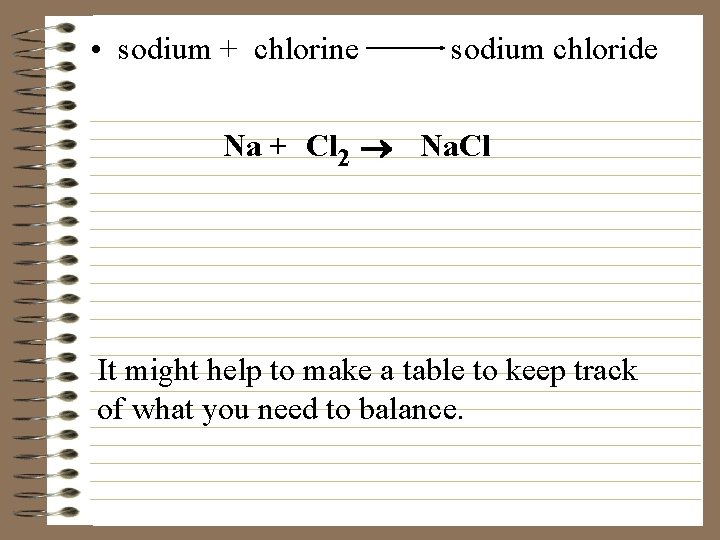
• sodium + chlorine sodium chloride Na + Cl 2 ® Na. Cl It might help to make a table to keep track of what you need to balance.
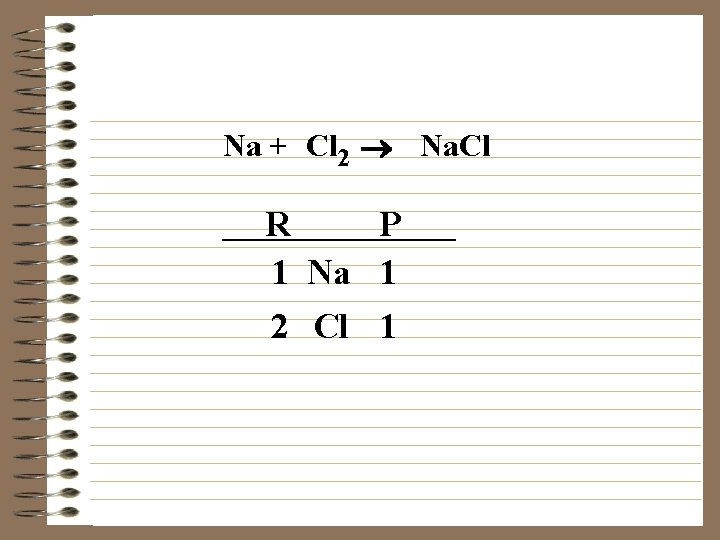
Na + Cl 2 ® Na. Cl R P 1 Na 1 2 Cl 1
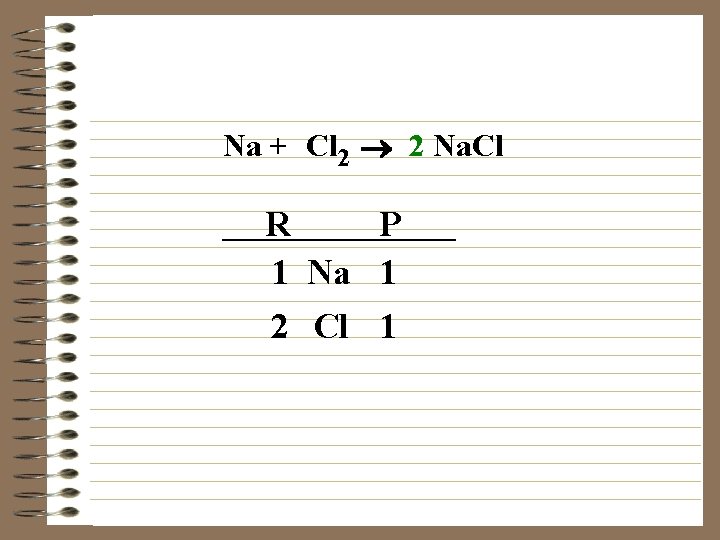
Na + Cl 2 ® 2 Na. Cl R P 1 Na 1 2 Cl 1
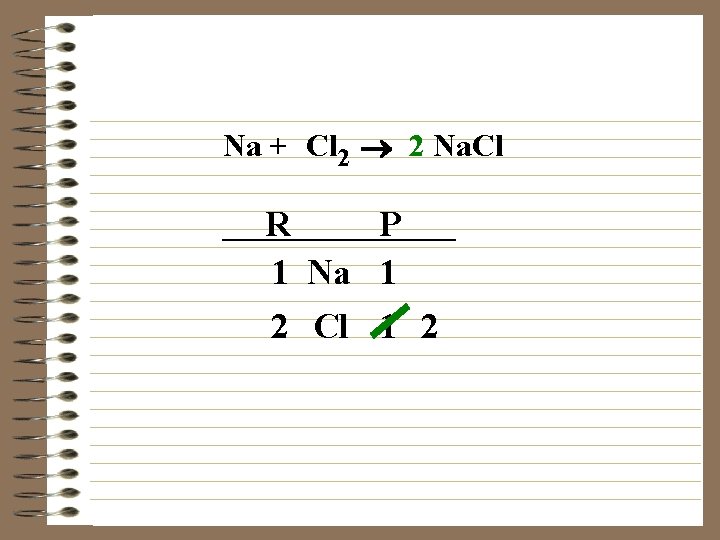
Na + Cl 2 ® 2 Na. Cl R P 1 Na 1 2 Cl 1 2
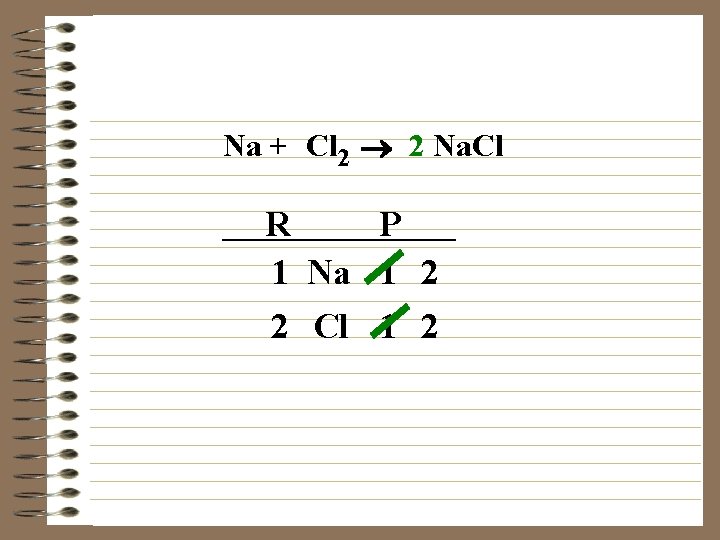
Na + Cl 2 ® 2 Na. Cl R P 1 Na 1 2 2 Cl 1 2
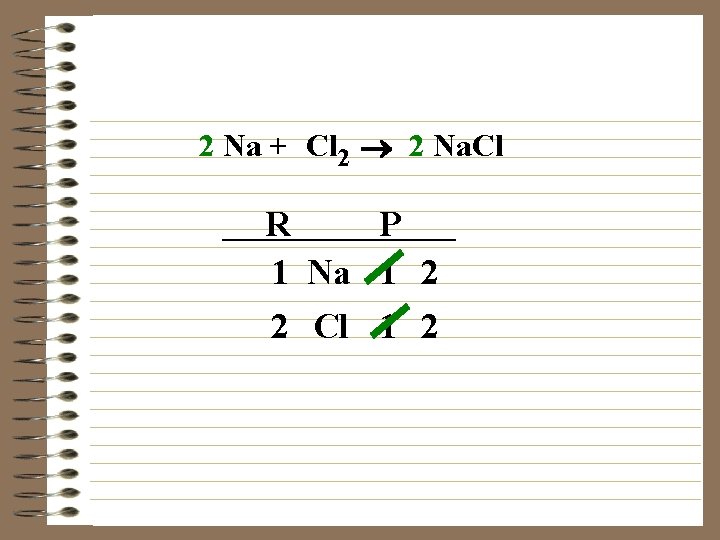
2 Na + Cl 2 ® 2 Na. Cl R P 1 Na 1 2 2 Cl 1 2
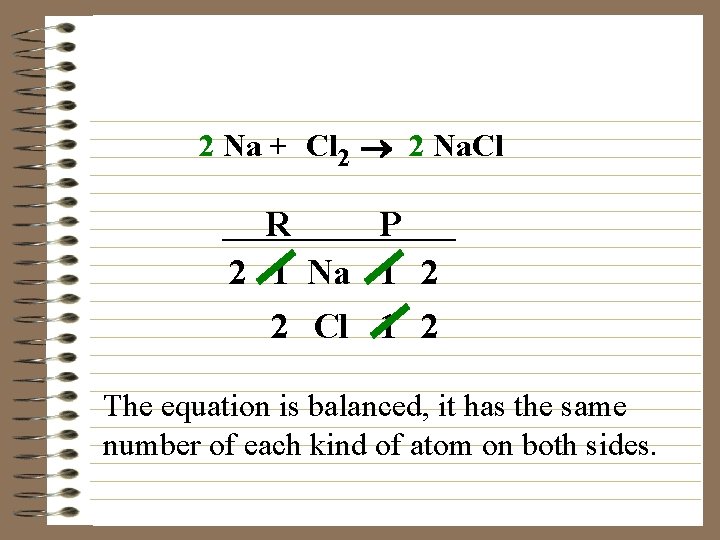
2 Na + Cl 2 ® 2 Na. Cl R P 2 1 Na 1 2 2 Cl 1 2 The equation is balanced, it has the same number of each kind of atom on both sides.
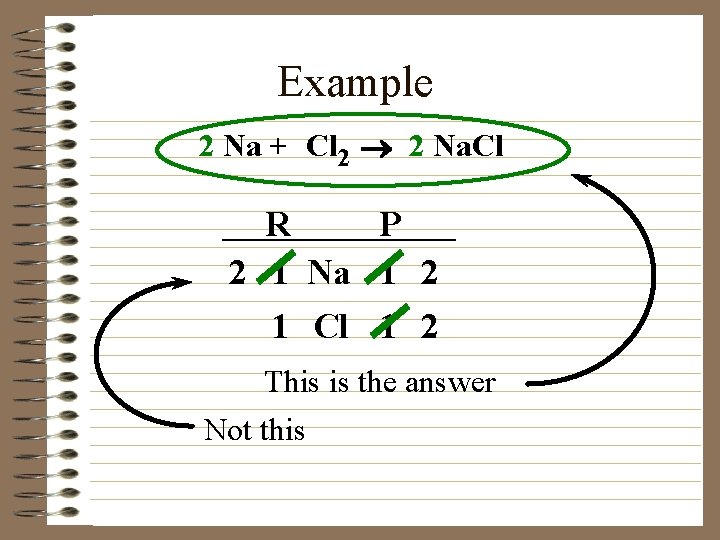
Example 2 Na + Cl 2 ® 2 Na. Cl R P 2 1 Na 1 2 1 Cl 1 2 This is the answer Not this
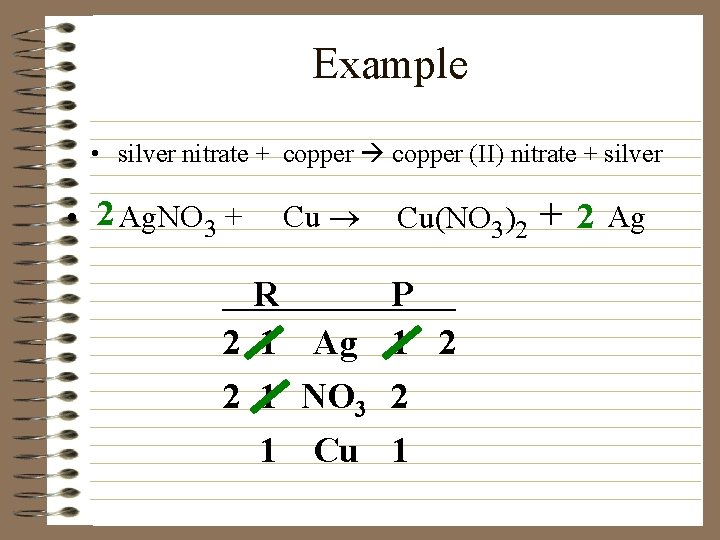
Example • silver nitrate + copper (II) nitrate + silver • 2 Ag. NO 3 + Cu ® Cu(NO 3)2 R P 2 1 Ag 1 2 2 1 NO 3 2 1 Cu 1 + 2 Ag
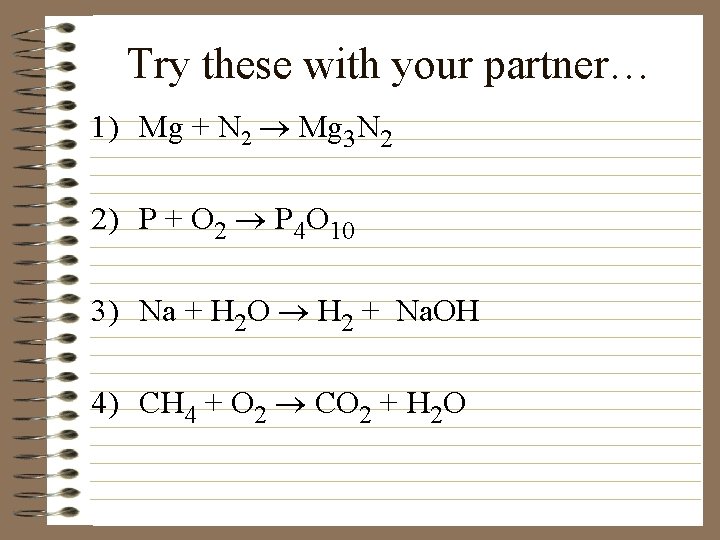
Try these with your partner… 1) Mg + N 2 ® Mg 3 N 2 2) P + O 2 ® P 4 O 10 3) Na + H 2 O ® H 2 + Na. OH 4) CH 4 + O 2 ® CO 2 + H 2 O
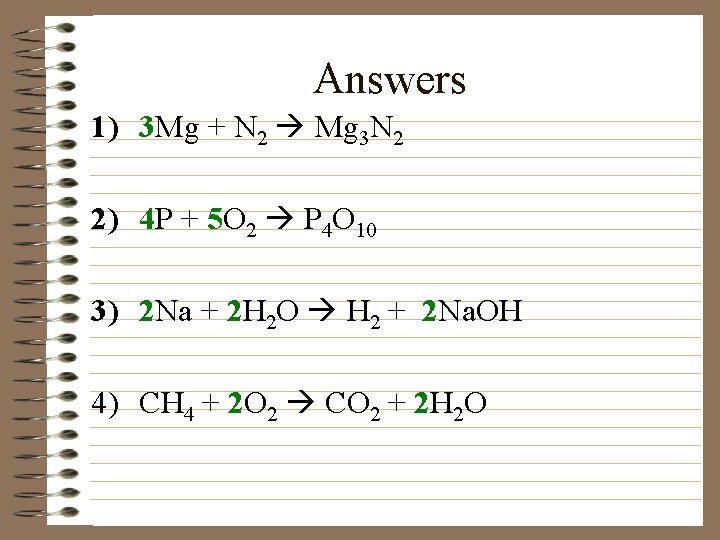
Answers 1) 3 Mg + N 2 Mg 3 N 2 2) 4 P + 5 O 2 P 4 O 10 3) 2 Na + 2 H 2 O H 2 + 2 Na. OH 4) CH 4 + 2 O 2 CO 2 + 2 H 2 O
- Slides: 26