Balancing Chemical Equations Chemistry Law of Conservation of

Balancing Chemical Equations Chemistry
Law of Conservation of Matter ● ● ● The mass (the amount of matter) must stay the same on the left and right side of a chemical reaction. Let's look at the combustion of CH 4 + O 2 → CO 2 + H 2 O What is the number of each element on each side?
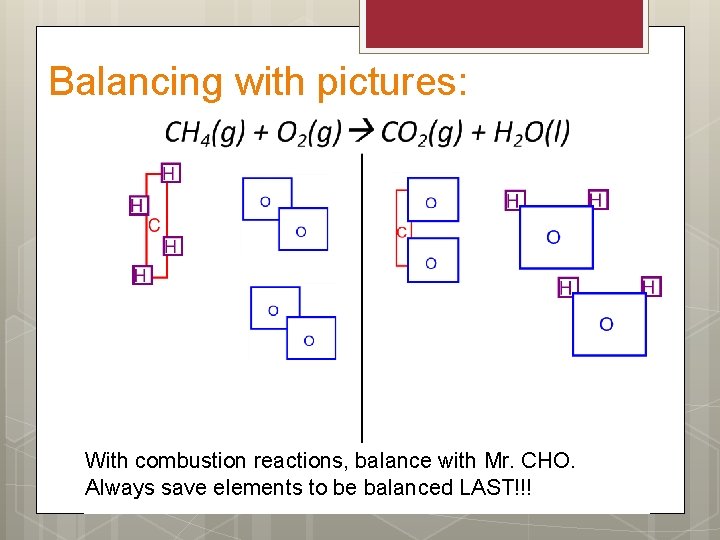
Balancing with pictures: With combustion reactions, balance with Mr. CHO. Always save elements to be balanced LAST!!!
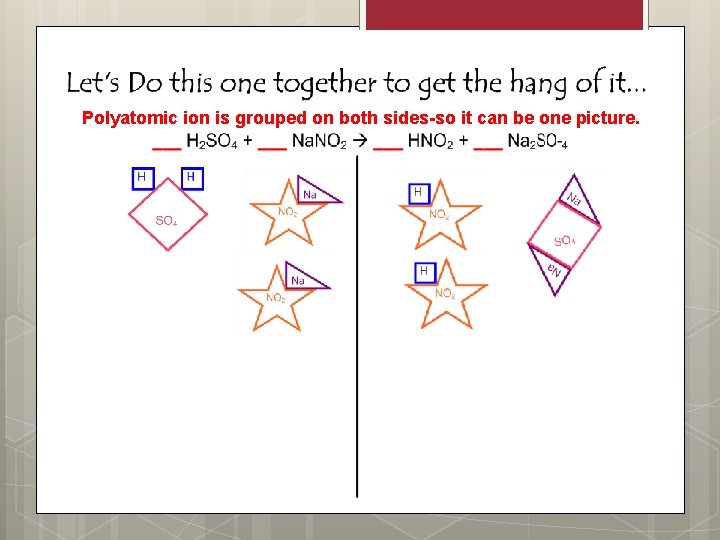
Polyatomic ion is grouped on both sides-so it can be one picture.

Final Hints ● Using the same process (drawing pictures), group up and determine how to demonstrate the balancing of YOUR questions. Hints: 1. Polyatomic Ions can be looked at as a UNIT (don't reak them up) IF they appear on BOTH sides of the equation. . . 2. ake a chart. Cut out pieces if you have to/draw out the pictures. Remember that you HAVE to add the ENTIRE compound-NEVER individual elements. . . 3. alance elemental (LONE) items last, ie: Na, O 2, etc. . . 4. EVER FORGET THE COEFFICIENTS. . . This is the final step in a chemical equation.

Breaking off… ● ● You will be counted off 1 -7. These are your group numbers. The problems that you are assigned to determine your problem numbers…for example, if you are #3, your numbers are 3, 10, and 17. Take your group number, that is your first problem, then add 7 to each up to #21. You will make posters for each of your groups. Get creative. Due by the end of the hour. Use the colored pencils, paper, etc.
- Slides: 6