Balancing chemical equations Chemical Reaction Reactant Product Chemical
Balancing chemical equations Chemical Reaction Reactant Product Chemical Equation Coefficient

Chemical reactions are represented by balanced chemical equations.

Conservation of Mass • The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction, it is conserved. • The mass of the reactants equals the mass of the products. massreactants = massproducts

Chemical Reactions • The process by which one or more substances are rearranged to form different substances is called a chemical reaction. What are some examples of evidence of a chemical reaction?

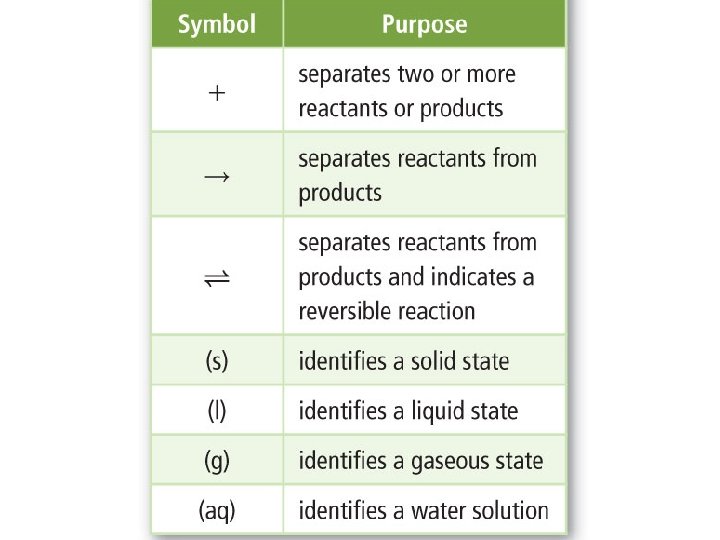
Representing Chemical Reactions • Chemists use statements called equations to represent chemical reactions. • Reactants: are the starting substances. • Products: are the substances formed in the reaction. • This table summarizes the symbols used in chemical equations.
Representing Chemical Reactions (cont. ) • In word equations, aluminum(s) + bromine(l) → aluminum bromide(s) reads as “aluminum and bromine react to produce aluminum bromide”. • Skeleton equations use symbols and formulas to represent the reactants and products. Example: Al(s) + Br(l) → Al. Br 3(s) Skeleton equations lack information about how many atoms are involved in the reaction.
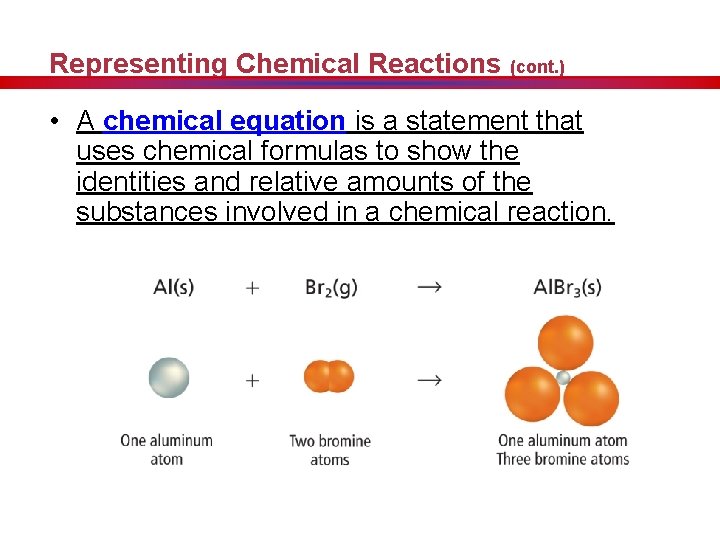
Representing Chemical Reactions (cont. ) • A chemical equation is a statement that uses chemical formulas to show the identities and relative amounts of the substances involved in a chemical reaction.
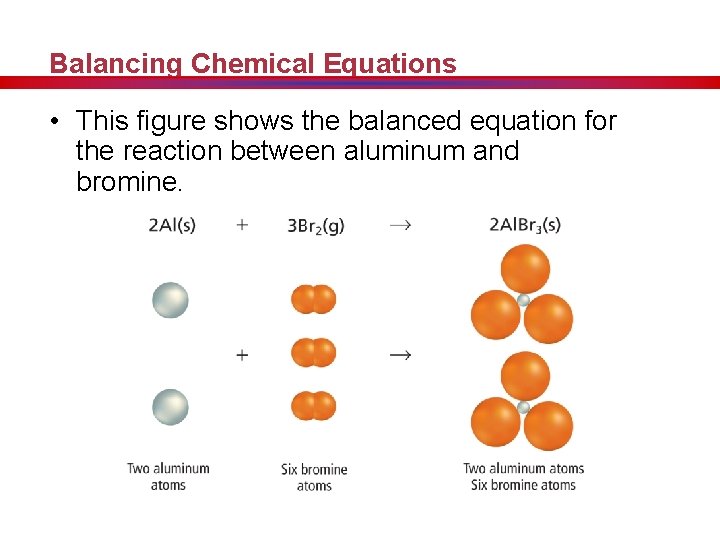
Balancing Chemical Equations • This figure shows the balanced equation for the reaction between aluminum and bromine.
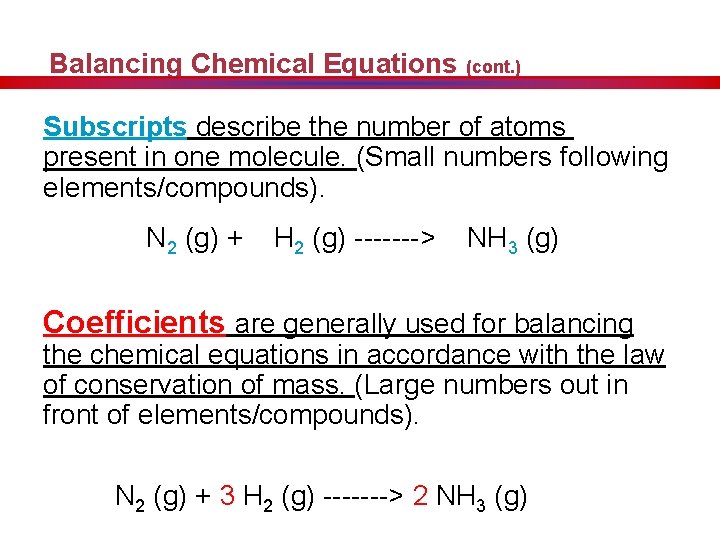
Balancing Chemical Equations (cont. ) Subscripts describe the number of atoms present in one molecule. (Small numbers following elements/compounds). N 2 (g) + H 2 (g) -------> NH 3 (g) Coefficients are generally used for balancing the chemical equations in accordance with the law of conservation of mass. (Large numbers out in front of elements/compounds). N 2 (g) + 3 H 2 (g) -------> 2 NH 3 (g)

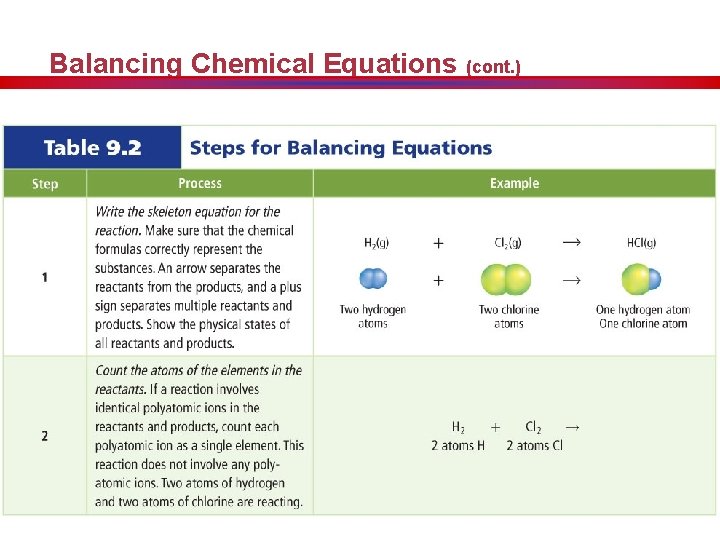
Balancing Chemical Equations (cont. )
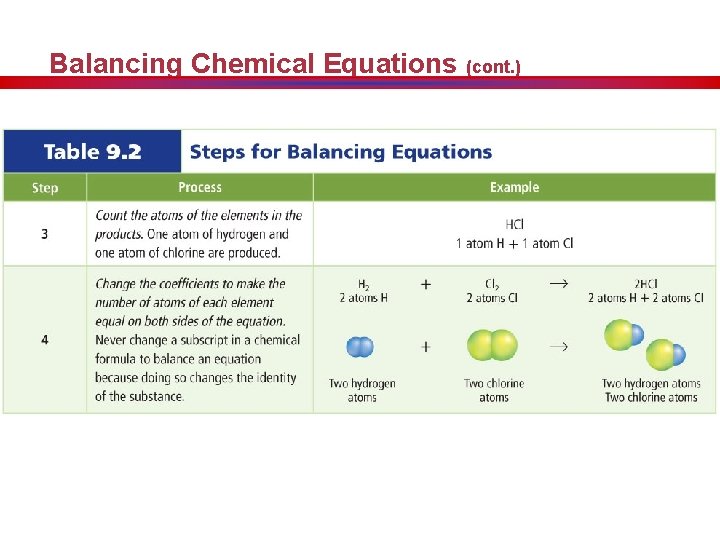
Balancing Chemical Equations (cont. )
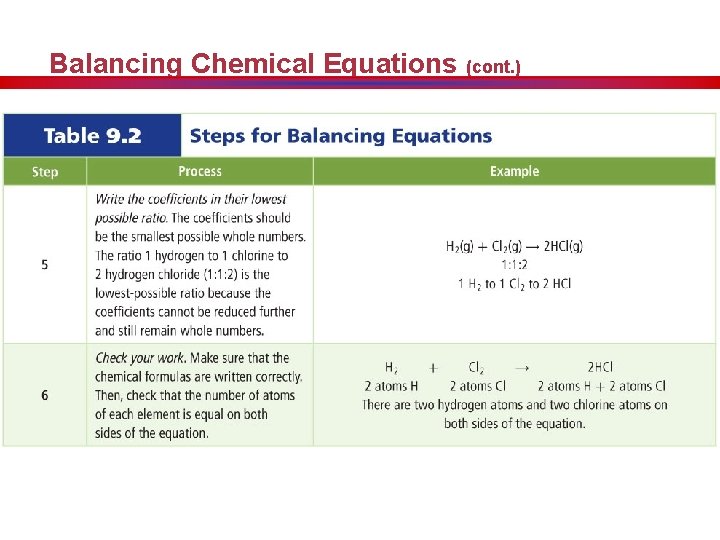
Balancing Chemical Equations (cont. )
Balancing Chemical Equations (cont. ) • The most fundamental law in chemistry is the law of conservation of mass. • Balanced equations show this law.
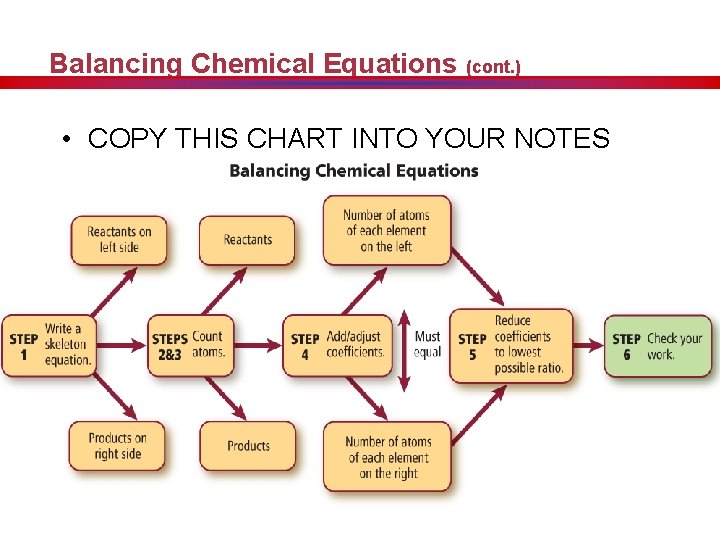
Balancing Chemical Equations (cont. ) • COPY THIS CHART INTO YOUR NOTES

Balancing Chemical Equations (cont. ) Two helpful hints 1) Never start with balancing oxygen; you can get into a crazy never ending loop. 2) If a polyatomic ion exists on both sides of the equation you can keep it together if balancing is giving you trouble. • Polyatomic ions are on the back of the periodic table.
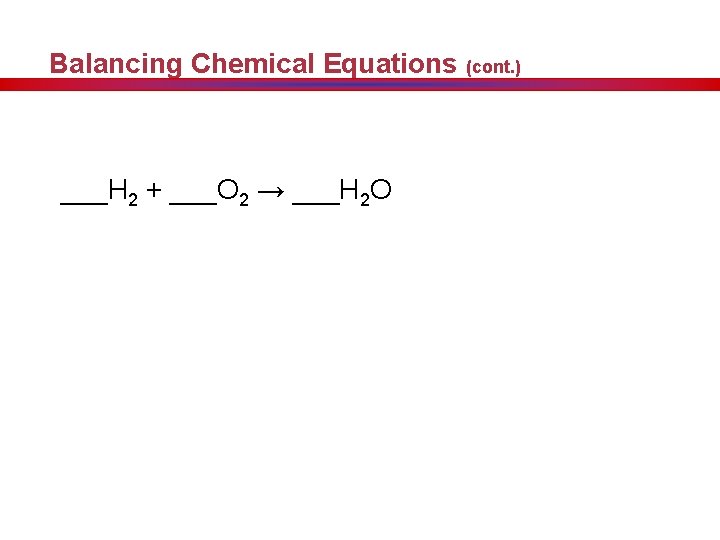
Balancing Chemical Equations (cont. ) ___H 2 + ___O 2 → ___H 2 O
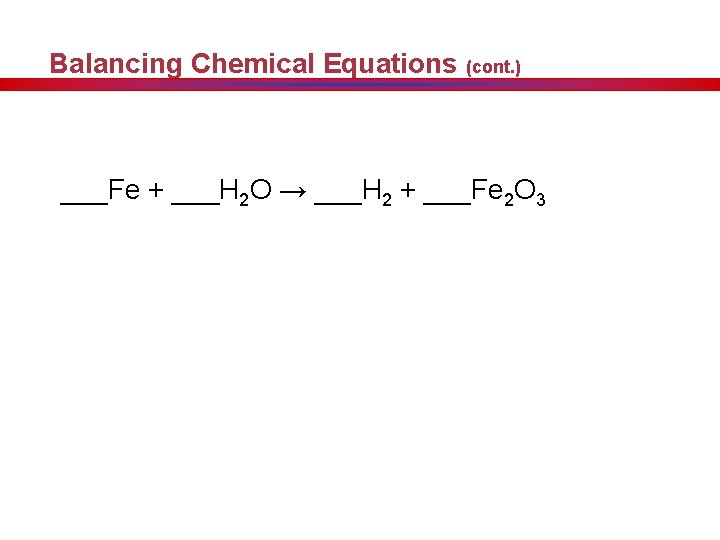
Balancing Chemical Equations (cont. ) ___Fe + ___H 2 O → ___H 2 + ___Fe 2 O 3
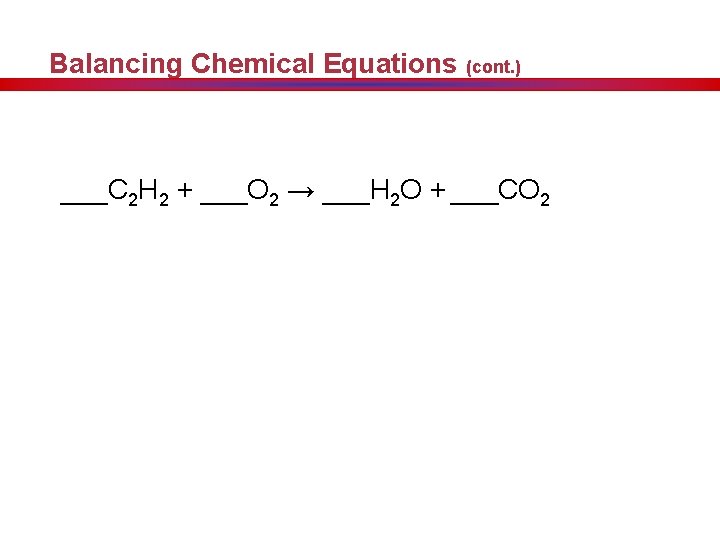
Balancing Chemical Equations (cont. ) ___C 2 H 2 + ___O 2 → ___H 2 O + ___CO 2
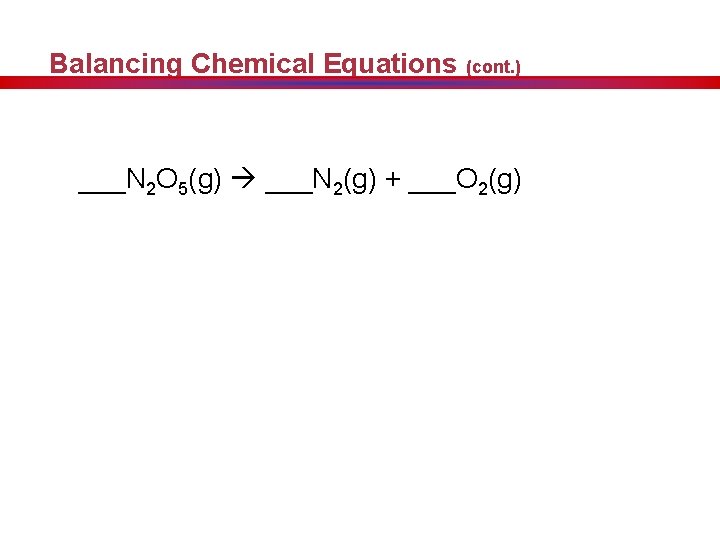
Balancing Chemical Equations (cont. ) ___N 2 O 5(g) ___N 2(g) + ___O 2(g)
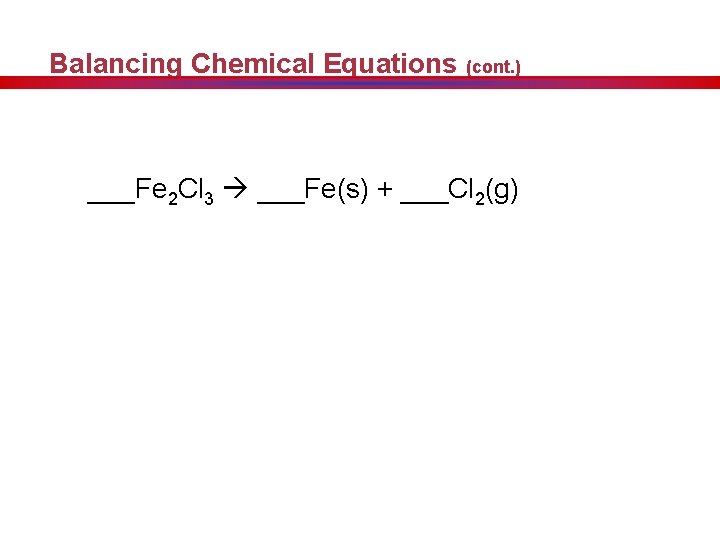
Balancing Chemical Equations (cont. ) ___Fe 2 Cl 3 ___Fe(s) + ___Cl 2(g)

Answer questions in the closure section of your bell ringer sheet.

Assessment Which of the following is NOT a chemical reaction? A. a piece of wood burning B. a car rusting C. an ice cube melting into water D. red litmus paper turning blue A. B. C. D. A B C D
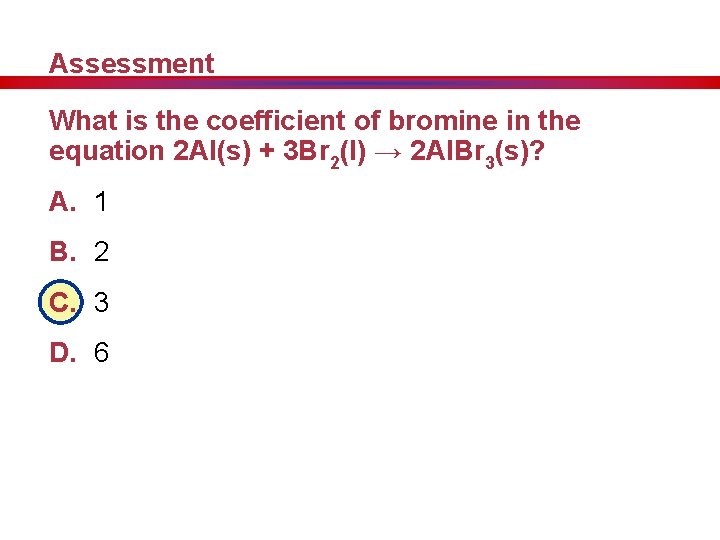
Assessment What is the coefficient of bromine in the equation 2 Al(s) + 3 Br 2(l) → 2 Al. Br 3(s)? A. 1 B. 2 C. 3 D. 6 A. B. C. D. A B C D
- Slides: 25