Balancing Chemical Equations Balanced Equation n Atoms cant

Balancing Chemical Equations

Balanced Equation n Atoms can’t be created or destroyed n Law of Conservation of Mass All the atoms we start with we must end up with n A balanced equation has the same number of each element on both sides of the equation (reactant and product sides). n
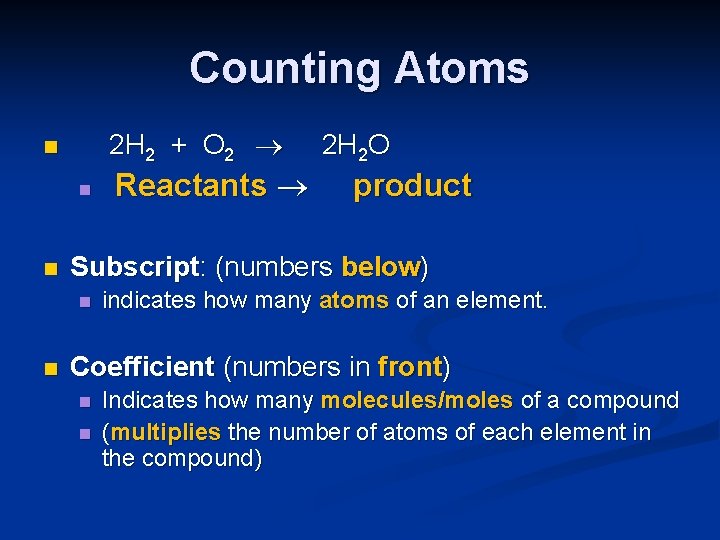
Counting Atoms 2 H 2 + O 2 n n n product Subscript: (numbers below) n n Reactants 2 H 2 O indicates how many atoms of an element. Coefficient (numbers in front) n n Indicates how many molecules/moles of a compound (multiplies the number of atoms of each element in the compound)

Chemical Formula Counting up Atoms
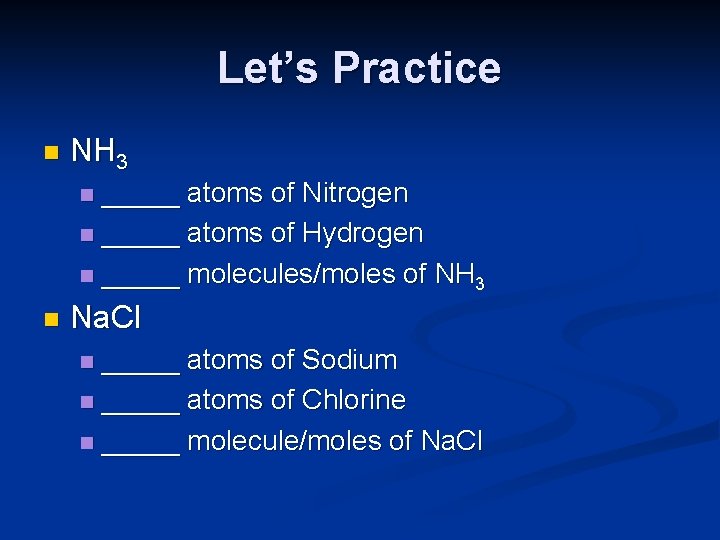
Let’s Practice n NH 3 _____ atoms of Nitrogen n _____ atoms of Hydrogen n _____ molecules/moles of NH 3 n n Na. Cl _____ atoms of Sodium n _____ atoms of Chlorine n _____ molecule/moles of Na. Cl n
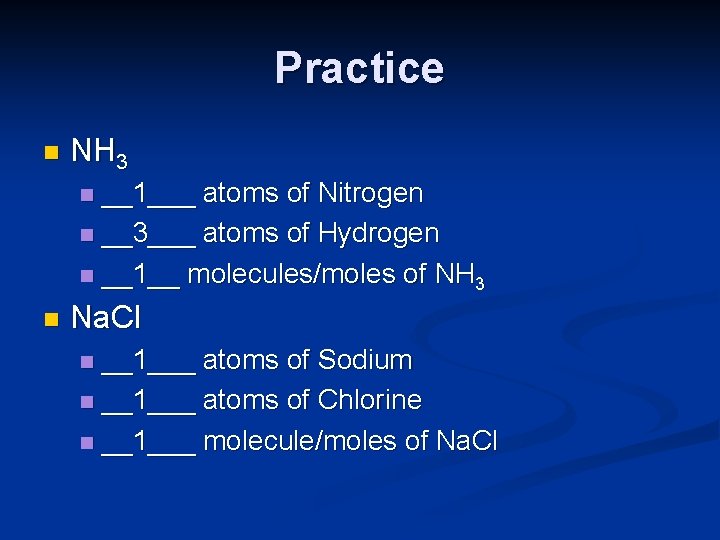
Practice n NH 3 __1___ atoms of Nitrogen n __3___ atoms of Hydrogen n __1__ molecules/moles of NH 3 n n Na. Cl __1___ atoms of Sodium n __1___ atoms of Chlorine n __1___ molecule/moles of Na. Cl n

Practice Again n 8 Na. Cl n n 3 NH 3 n n _____ atoms of Sodium _____ atoms of Chlorine _____ molecule/moles of Na. Cl ____ atoms of Nitrogen ____ atoms of Hydrogen ___ molecule/moles of NH 3 Ca(NO 3)2 n n _____ atoms of Calcium _____ atoms of Nitrogen _____ atoms of Oxygen _____molecule/moles of Ca(NO 3)2

Practice Again n 8 Na. Cl n n 3 NH 3 n n __8___ atoms of Sodium __8___ atoms of Chlorine __8___ molecule/moles of Na. Cl __3__ atoms of Nitrogen __9__ atoms of Hydrogen __3_ molecules/moles of NH 3 Ca(NO 3)2 n n n ___1__ atoms of Calcium ___2__ atoms of Nitrogen ___6__ atoms of Oxygen ___1__molecule/moles of Ca(NO 3)2 What if there is a coefficient of 2 in front of Ca(NO 3)2 ?
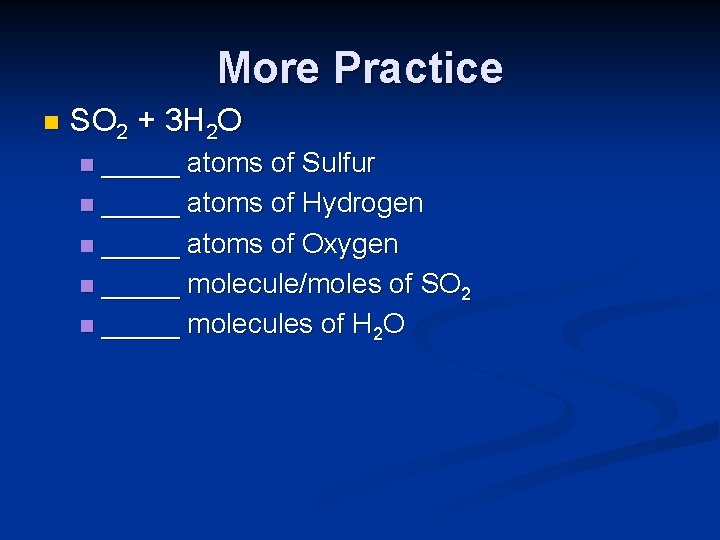
More Practice n SO 2 + 3 H 2 O _____ atoms of Sulfur n _____ atoms of Hydrogen n _____ atoms of Oxygen n _____ molecule/moles of SO 2 n _____ molecules of H 2 O n

More Practice n SO 2 + 3 H 2 O __1___ atoms of Sulfur n __6___ atoms of Hydrogen n __5___ atoms of Oxygen n __1___ molecule/moles of SO 2 n __3___ molecules of H 2 O n
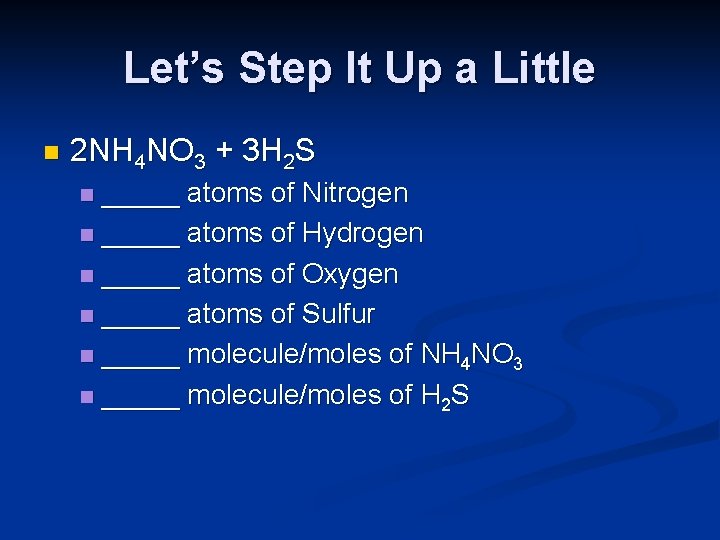
Let’s Step It Up a Little n 2 NH 4 NO 3 + 3 H 2 S _____ atoms of Nitrogen n _____ atoms of Hydrogen n _____ atoms of Oxygen n _____ atoms of Sulfur n _____ molecule/moles of NH 4 NO 3 n _____ molecule/moles of H 2 S n
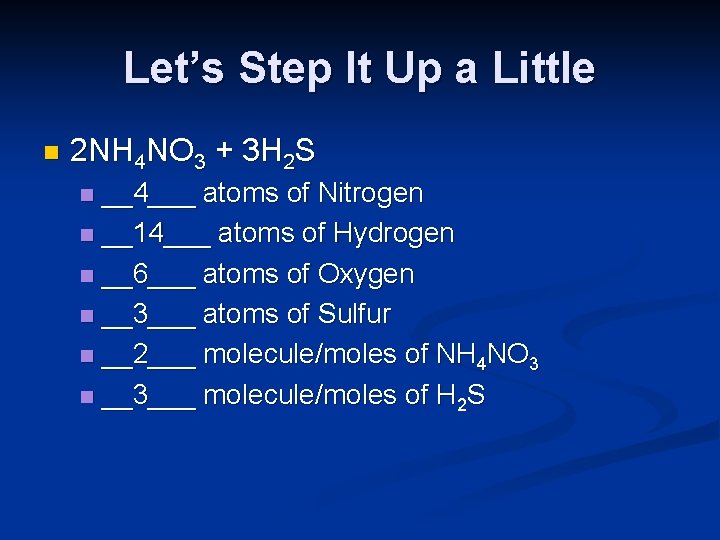
Let’s Step It Up a Little n 2 NH 4 NO 3 + 3 H 2 S __4___ atoms of Nitrogen n __14___ atoms of Hydrogen n __6___ atoms of Oxygen n __3___ atoms of Sulfur n __2___ molecule/moles of NH 4 NO 3 n __3___ molecule/moles of H 2 S n

You Must Be Joking! n 2 Na 2 CO 3 + 3 CO 2 + 4 Na. Cl + Mg(NO 3)2 n n n n n _____ atoms of Sodium _____ atoms of Carbon _____ atoms of Oxygen _____ atoms of Chlorine _____ atoms of Nitrogen _____ atoms of Magnesium _____molecules/moles of Na 2 CO 3 _____ molecule/moles of CO 2 _____ molecule/moles of Na. Cl _____ molecule/moles of Mg(NO 3)2

You Must Be Joking! n 2 Na 2 CO 3 + 3 CO 2 + 4 Na. Cl + Mg(NO 3)2 n n n n n __8___ atoms of Sodium __5___ atoms of Carbon __18___ atoms of Oxygen __4___ atoms of Chlorine __2___ atoms of Nitrogen __1___ atom of Magnesium __2___molecules/moles of Na 2 CO 3 __3___ molecule/moles of CO 2 __4___ molecule/moles of Na. Cl __1___ molecule/moles of Mg(NO 3)2

C + O O ® O C C + O 2 CO 2 n This equation is already balanced n What if it isn’t already? n O

C + O O ® C O C + O 2 CO n We need one more oxygen in the products. n Can’t change the formula, because it describes what is produced. n Remember, oxygen is a diatomic molecule. n

C + O O ® C O The other Oxygen must be used to make another CO n But where did the other C come from? n
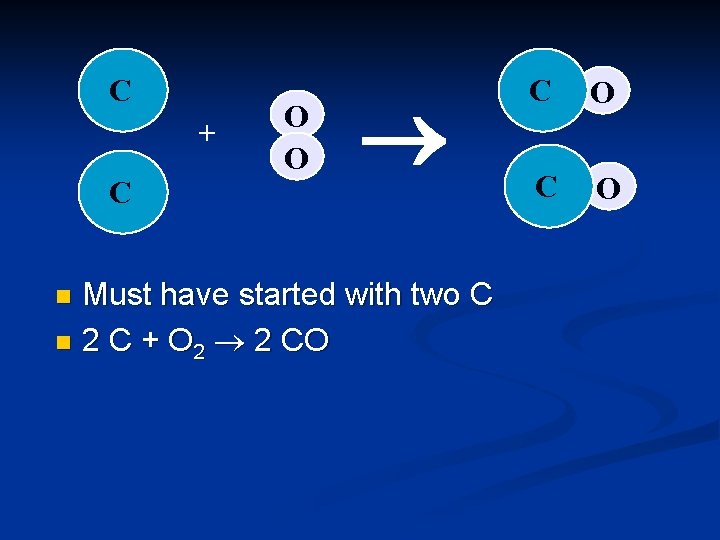
C + C O O ® Must have started with two C n 2 C + O 2 2 CO n C O

Rules for balancing 1 Write the correct formulas for all the reactants and products 2 Count the number of atoms of each type appearing on both sides 3 Balance the elements (make them same amount on reactant and product sides) one at a time by adding coefficients (the numbers in front). 4 Check to make sure it is balanced.

Never! n Change a subscript to balance an equation. If you change the formula you are describing a different reaction. n H 2 O is a different compound than H 2 O 2 n n Never put a coefficient in the middle of a formula n 2 Na. Cl is okay, Na 2 Cl is not.

Example H 2 + O 2 ® H 2 O Make a table to keep track of where you are at

Example H 2 + O 2 ® H 2 O R P 2 H 2 2 O 1 Need twice as much O in the product
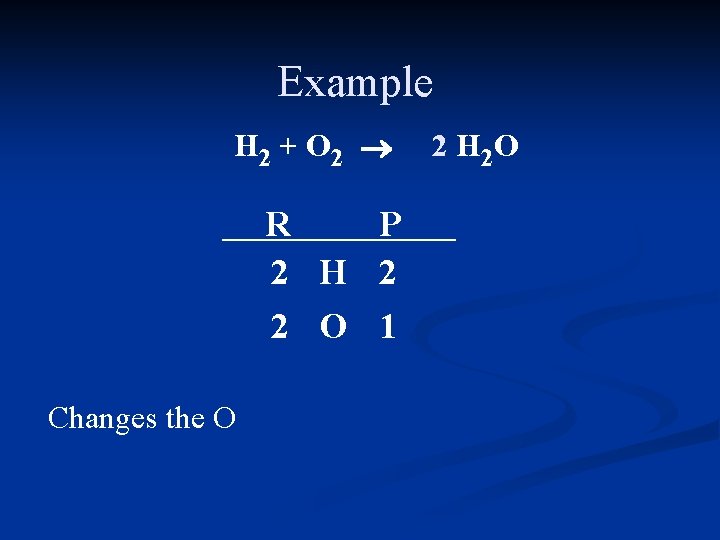
Example H 2 + O 2 ® R P 2 H 2 2 O 1 Changes the O 2 H 2 O
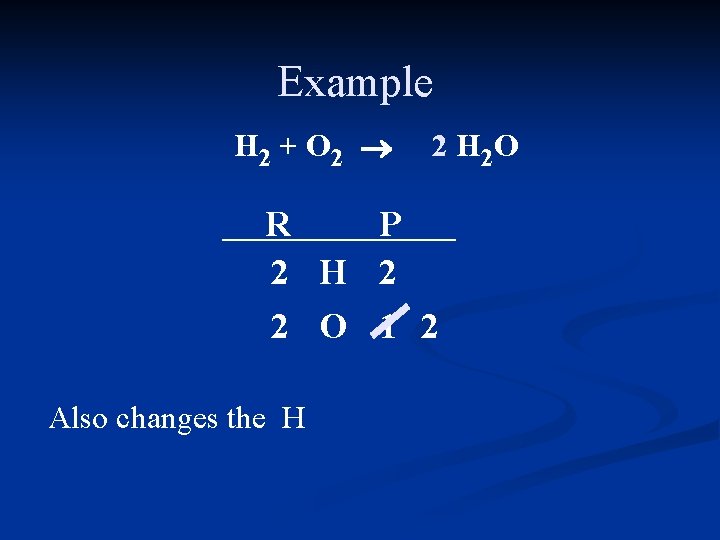
Example H 2 + O 2 ® 2 H 2 O R P 2 H 2 2 O 1 2 Also changes the H
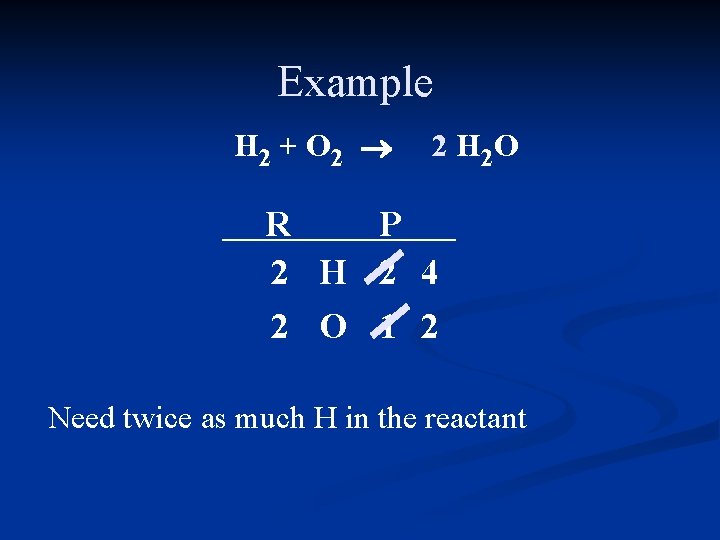
Example H 2 + O 2 ® 2 H 2 O R P 2 H 2 4 2 O 1 2 Need twice as much H in the reactant

Example 2 H 2 + O 2 ® 2 H 2 O R P 2 H 2 4 2 O 1 2 Recount
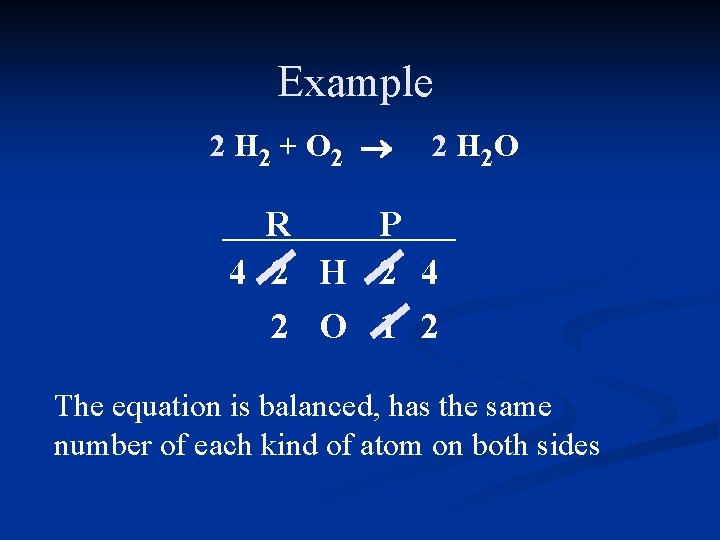
Example 2 H 2 + O 2 ® 2 H 2 O R P 4 2 H 2 4 2 O 1 2 The equation is balanced, has the same number of each kind of atom on both sides
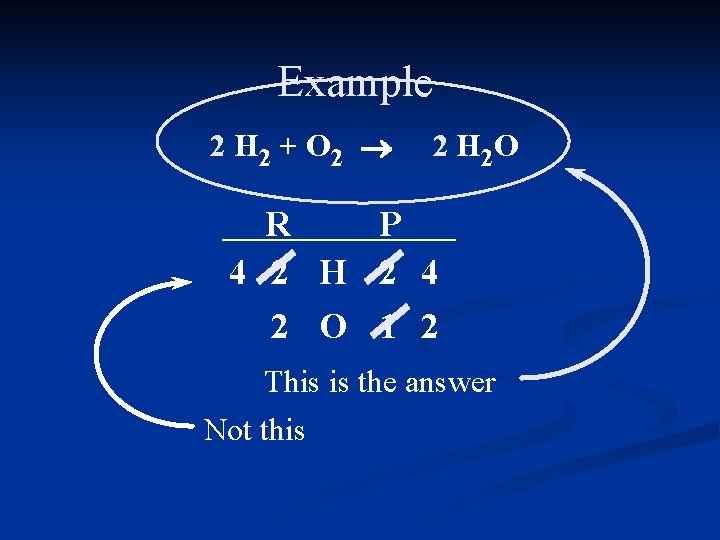
Example 2 H 2 + O 2 ® 2 H 2 O R P 4 2 H 2 4 2 O 1 2 This is the answer Not this
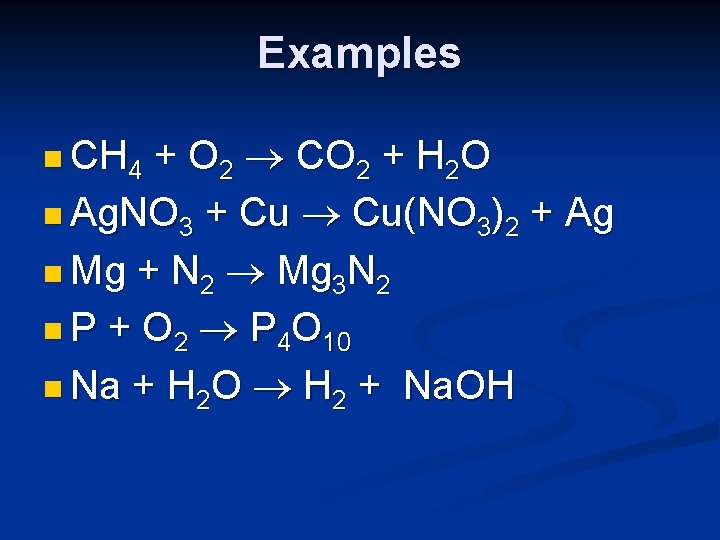
Examples + O 2 CO 2 + H 2 O n Ag. NO 3 + Cu Cu(NO 3)2 + Ag n Mg + N 2 Mg 3 N 2 n P + O 2 P 4 O 10 n Na + H 2 O H 2 + Na. OH n CH 4
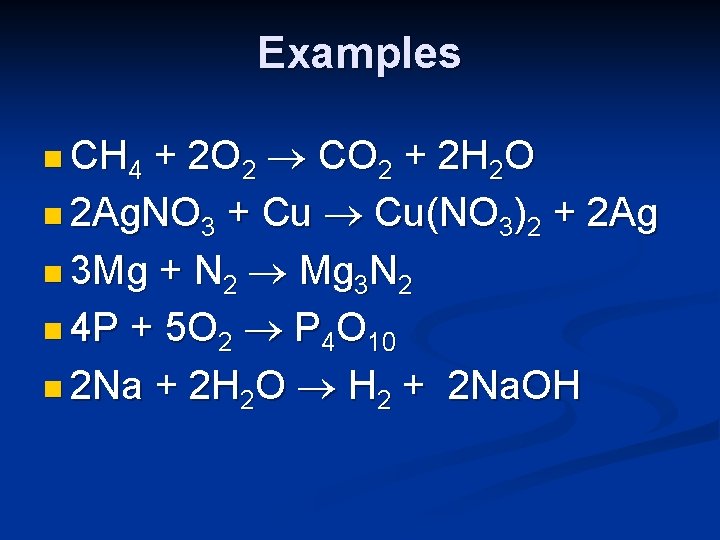
Examples + 2 O 2 CO 2 + 2 H 2 O n 2 Ag. NO 3 + Cu Cu(NO 3)2 + 2 Ag n 3 Mg + N 2 Mg 3 N 2 n 4 P + 5 O 2 P 4 O 10 n 2 Na + 2 H 2 O H 2 + 2 Na. OH n CH 4
- Slides: 30