Balancing Chemical Equations and Predicting Products https quizizz
Balancing Chemical Equations and Predicting Products https: //quizizz. com/admin/quiz/570 e 48157915303 f 704 a 466 b
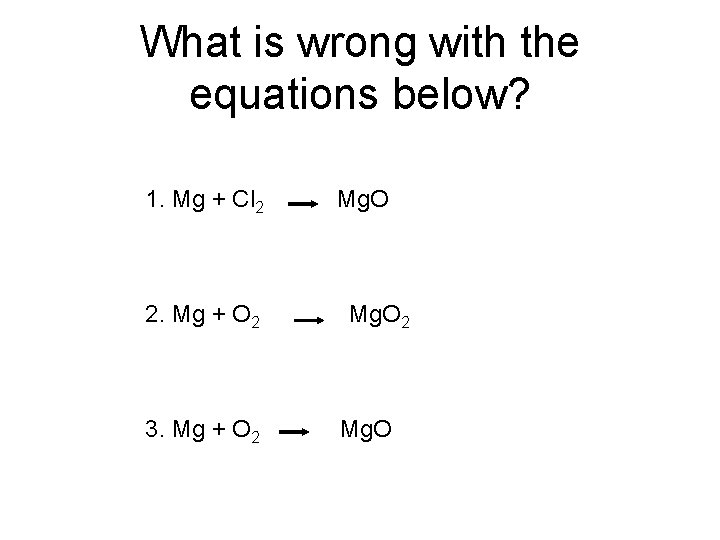
What is wrong with the equations below? 1. Mg + Cl 2 2. Mg + O 2 3. Mg + O 2 Mg. O
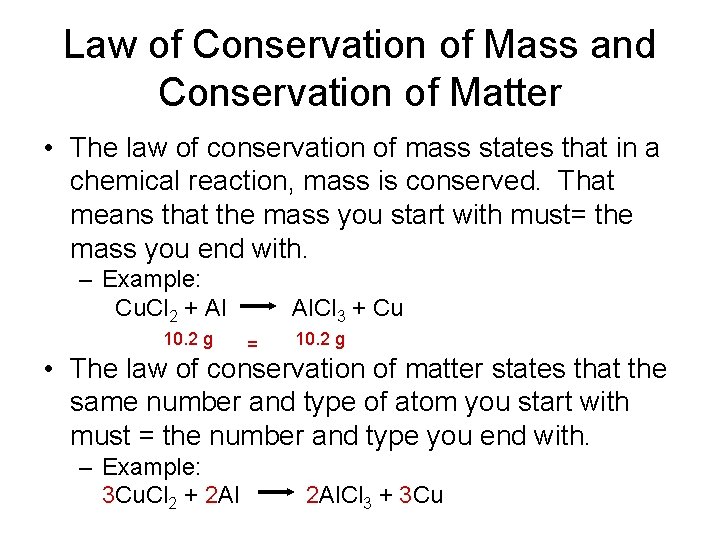
Law of Conservation of Mass and Conservation of Matter • The law of conservation of mass states that in a chemical reaction, mass is conserved. That means that the mass you start with must= the mass you end with. – Example: Cu. Cl 2 + Al 10. 2 g Al. Cl 3 + Cu = 10. 2 g • The law of conservation of matter states that the same number and type of atom you start with must = the number and type you end with. – Example: 3 Cu. Cl 2 + 2 Al. Cl 3 + 3 Cu

What must you remember when balancing chemical equations?
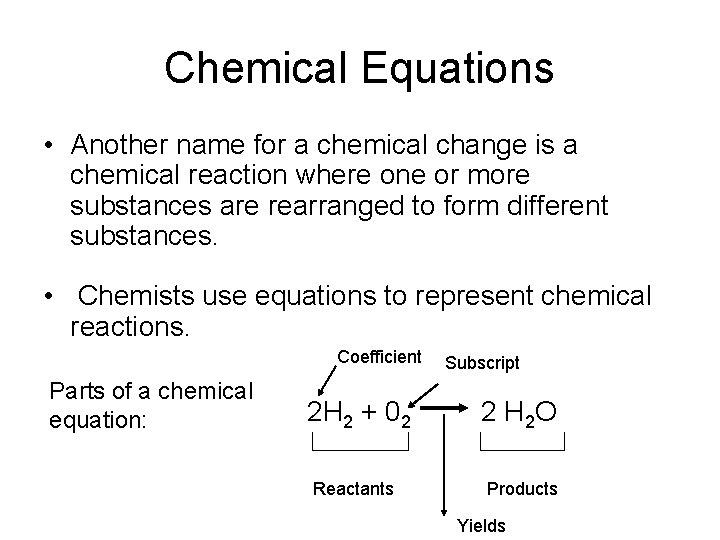
Chemical Equations • Another name for a chemical change is a chemical reaction where one or more substances are rearranged to form different substances. • Chemists use equations to represent chemical reactions. Coefficient Parts of a chemical equation: 2 H 2 + 02 Reactants Subscript 2 H 2 O Products Yields
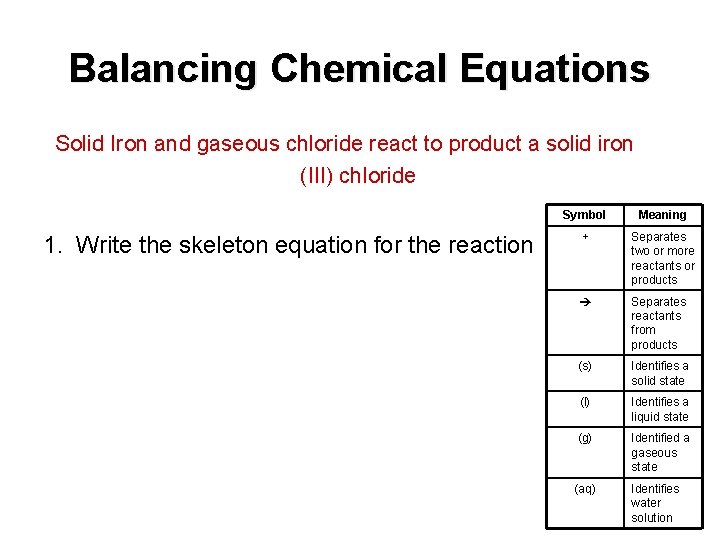
Balancing Chemical Equations Solid Iron and gaseous chloride react to product a solid iron (III) chloride 1. Write the skeleton equation for the reaction Symbol Meaning + Separates two or more reactants or products Separates reactants from products (s) Identifies a solid state (l) Identifies a liquid state (g) Identified a gaseous state (aq) Identifies water solution

Symbols for Equations Symbol Meaning + Separates two or more reactants or products (s) Separates reactants from products Identifies a solid state (l) Identifies a liquid state (g) Identified a gaseous state (aq) Identifies water solution
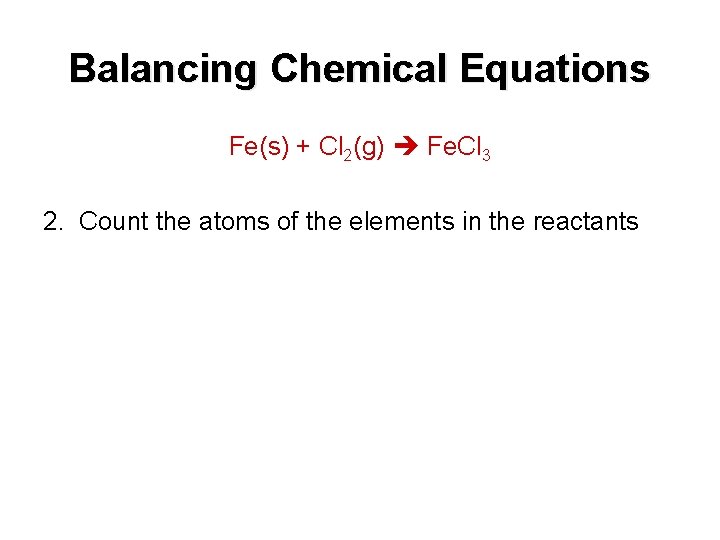
Balancing Chemical Equations Fe(s) + Cl 2(g) Fe. Cl 3 2. Count the atoms of the elements in the reactants
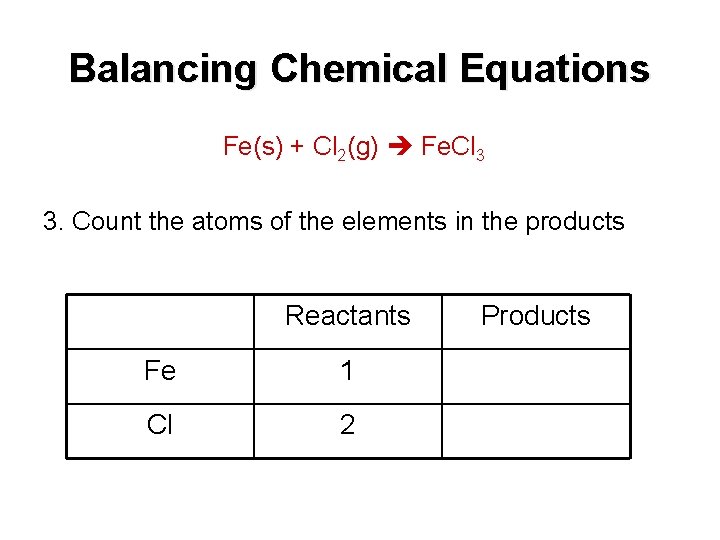
Balancing Chemical Equations Fe(s) + Cl 2(g) Fe. Cl 3 3. Count the atoms of the elements in the products Reactants Fe 1 Cl 2 Products
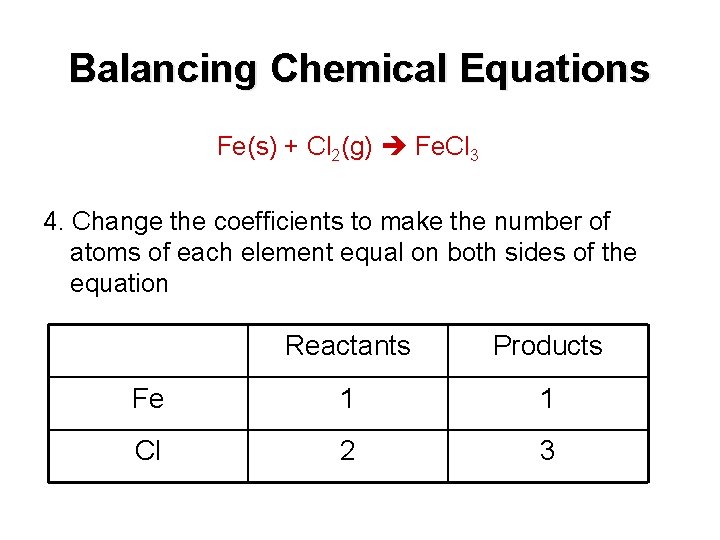
Balancing Chemical Equations Fe(s) + Cl 2(g) Fe. Cl 3 4. Change the coefficients to make the number of atoms of each element equal on both sides of the equation Reactants Products Fe 1 1 Cl 2 3
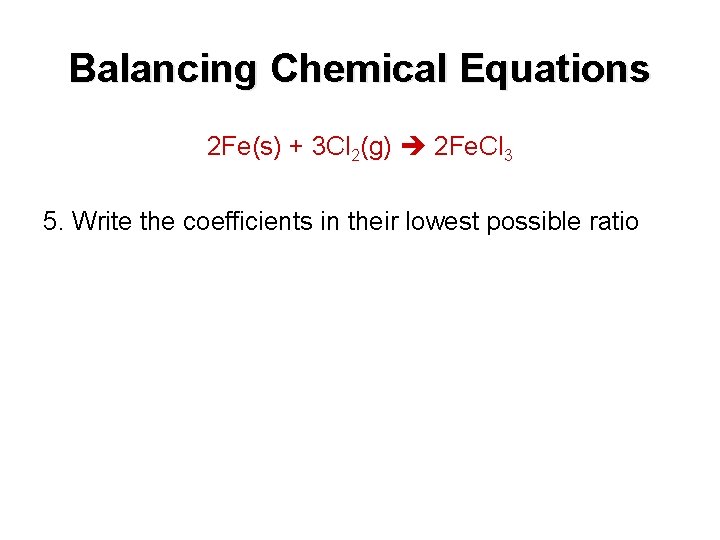
Balancing Chemical Equations 2 Fe(s) + 3 Cl 2(g) 2 Fe. Cl 3 5. Write the coefficients in their lowest possible ratio
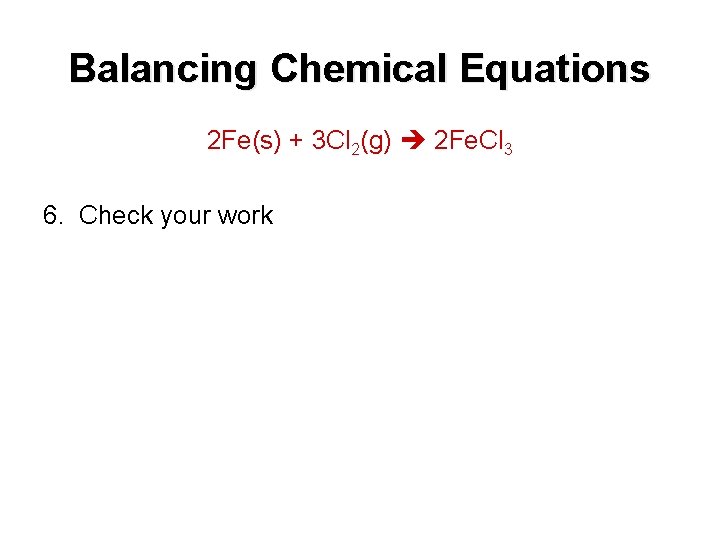
Balancing Chemical Equations 2 Fe(s) + 3 Cl 2(g) 2 Fe. Cl 3 6. Check your work
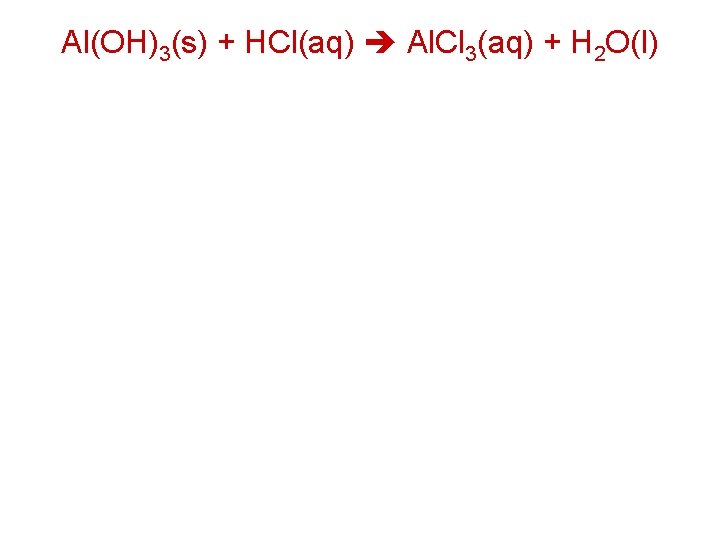
Al(OH)3(s) + HCl(aq) Al. Cl 3(aq) + H 2 O(l)
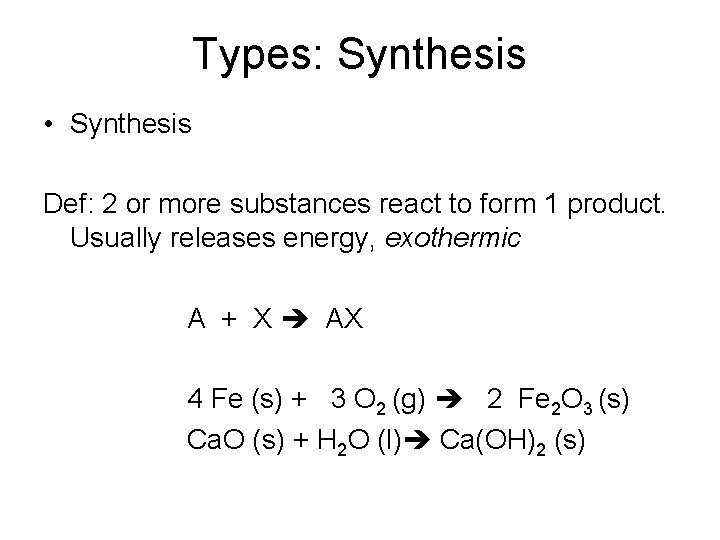
Types: Synthesis • Synthesis Def: 2 or more substances react to form 1 product. Usually releases energy, exothermic A + X AX 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) Ca. O (s) + H 2 O (l) Ca(OH)2 (s)
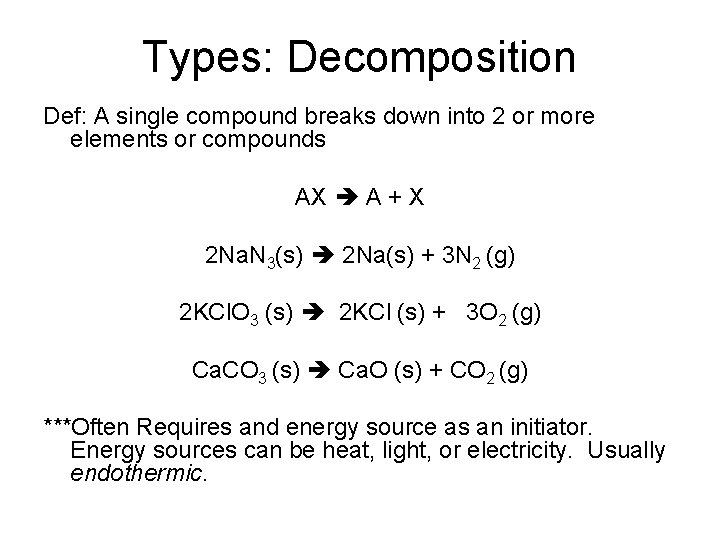
Types: Decomposition Def: A single compound breaks down into 2 or more elements or compounds AX A + X 2 Na. N 3(s) 2 Na(s) + 3 N 2 (g) 2 KCl. O 3 (s) 2 KCl (s) + 3 O 2 (g) Ca. CO 3 (s) Ca. O (s) + CO 2 (g) ***Often Requires and energy source as an initiator. Energy sources can be heat, light, or electricity. Usually endothermic.
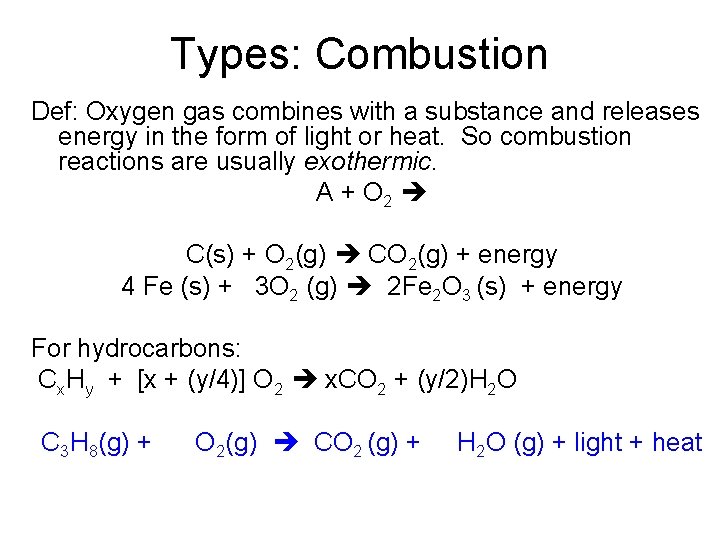
Types: Combustion Def: Oxygen gas combines with a substance and releases energy in the form of light or heat. So combustion reactions are usually exothermic. A + O 2 C(s) + O 2(g) CO 2(g) + energy 4 Fe (s) + 3 O 2 (g) 2 Fe 2 O 3 (s) + energy For hydrocarbons: Cx. Hy + [x + (y/4)] O 2 x. CO 2 + (y/2)H 2 O C 3 H 8(g) + O 2(g) CO 2 (g) + H 2 O (g) + light + heat
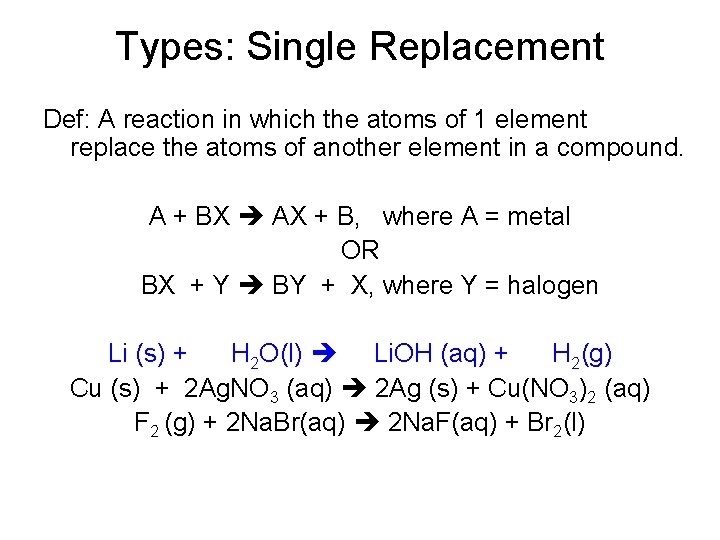
Types: Single Replacement Def: A reaction in which the atoms of 1 element replace the atoms of another element in a compound. A + BX AX + B, where A = metal OR BX + Y BY + X, where Y = halogen Li (s) + H 2 O(l) Li. OH (aq) + H 2(g) Cu (s) + 2 Ag. NO 3 (aq) 2 Ag (s) + Cu(NO 3)2 (aq) F 2 (g) + 2 Na. Br(aq) 2 Na. F(aq) + Br 2(l)
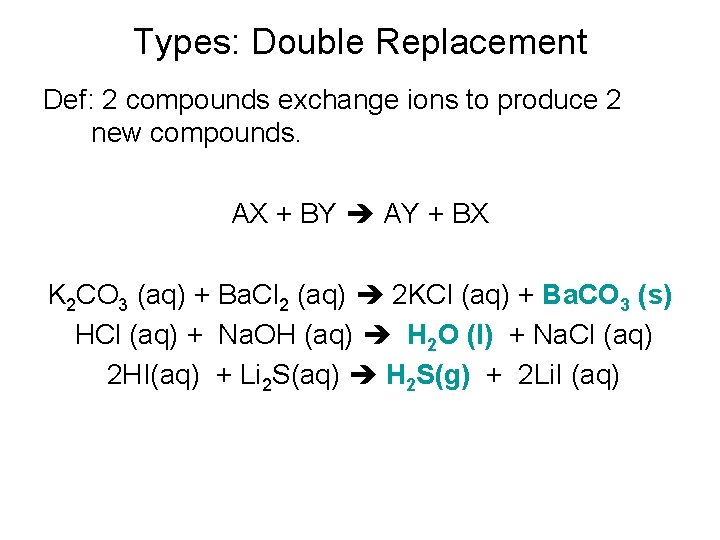
Types: Double Replacement Def: 2 compounds exchange ions to produce 2 new compounds. AX + BY AY + BX K 2 CO 3 (aq) + Ba. Cl 2 (aq) 2 KCl (aq) + Ba. CO 3 (s) HCl (aq) + Na. OH (aq) H 2 O (l) + Na. Cl (aq) 2 HI(aq) + Li 2 S(aq) H 2 S(g) + 2 Li. I (aq)

Predicting Products Synthesis Solid magnesium reacts with oxygen gas to produce? Solid iron and chlorine gas react to produce? Fe(III) product
Predicting Products Decomposition Magnesium bromide Cobalt (II) oxide

Predicting Products Combustion Aqueous ethanol (C 2 H 6 OH) reacts with oxygen to produce? Solid barium reacts with oxygen to produce?
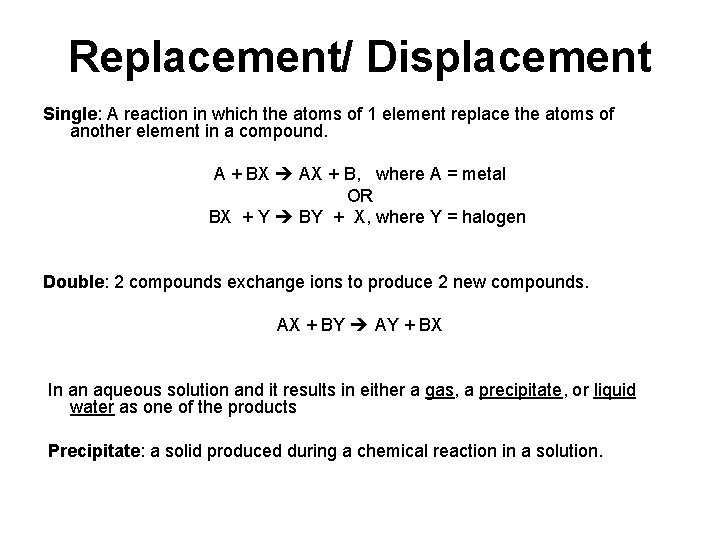
Replacement/ Displacement Single: A reaction in which the atoms of 1 element replace the atoms of another element in a compound. A + BX AX + B, where A = metal OR BX + Y BY + X, where Y = halogen Double: 2 compounds exchange ions to produce 2 new compounds. AX + BY AY + BX In an aqueous solution and it results in either a gas, a precipitate, or liquid water as one of the products Precipitate: a solid produced during a chemical reaction in a solution.
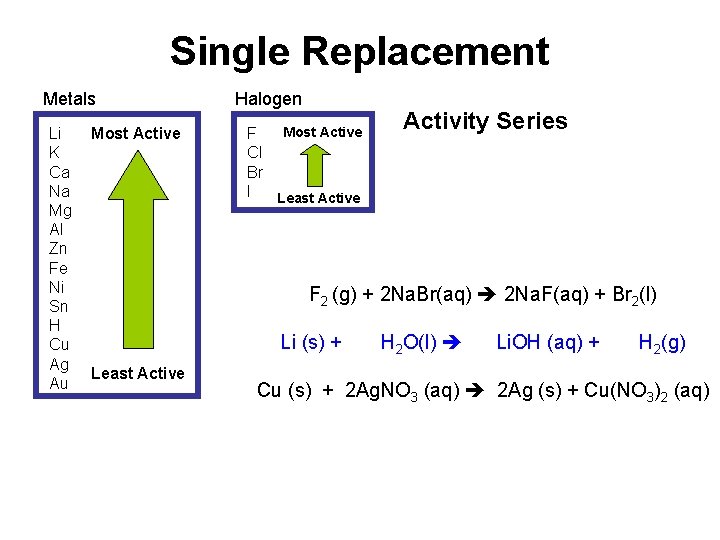
Single Replacement Metals Li K Ca Na Mg Al Zn Fe Ni Sn H Cu Ag Au Most Active Halogen Most Active F Cl Br I Least Active Activity Series F 2 (g) + 2 Na. Br(aq) 2 Na. F(aq) + Br 2(l) Li (s) + Least Active H 2 O(l) Li. OH (aq) + H 2(g) Cu (s) + 2 Ag. NO 3 (aq) 2 Ag (s) + Cu(NO 3)2 (aq)
Predicting Products Single Replacement Iron metal reacts with copper (II) sulfate to yield? Liquid Bromine reacts with magnesium chloride to yield? Magnesium metal reacts with potassium chloride to yield?

Predicting Products Double Replacement Calcium Iodide reacts with mercury (II) nitrate to yield? Sulfuric acid reacts with Aluminum hydroxide to yield? Ammonium chloride reacts with silver sulfate to yield?
- Slides: 25