BALANCING CHEMICAL EQUATIONS According to the Law of

BALANCING CHEMICAL EQUATIONS

According to the Law of Conservation of Matter, atoms cannot be created or destroyed. Therefore, matter is conserved and atoms can only be re-arranged to form new substances. The type and amount of atoms cannot change.

WRITING CHEMICAL FORMULAS To Write a Formula: 1. Write the symbols of the substances to be bonded. 2. Indicate the oxidation number of each substance. 3. Determine what multipliers are needed to equalize the charge. 4. Write the multiplier after each substance. 5. Write the positive substance first, the negative one second. Example: Calcium and Nitrate 1. Calcium - Ca; nitrate = NO 3 2. Ca = +2; NO 3 = -1 3. Because 2 X -1 = 2, The multiplier for this formula is 2. 4. The subscripts are 1 and 2; Ca, and (NO 3)2. 5. Ca (NO 3)2 , or calcium nitrate.
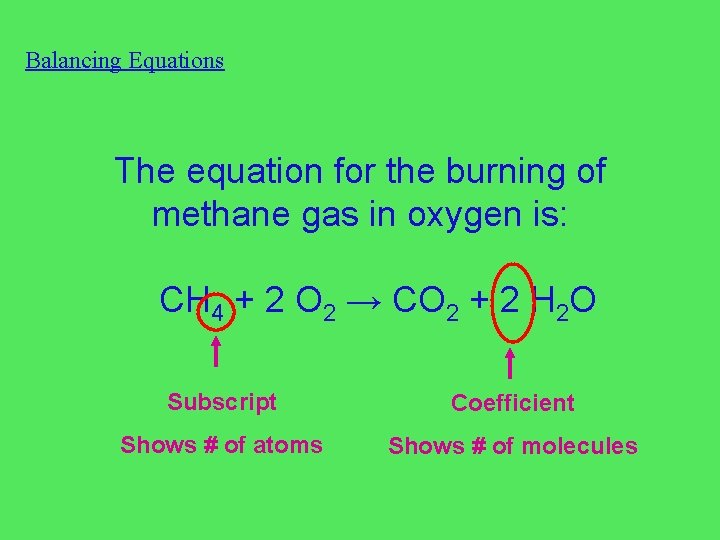
Balancing Equations The equation for the burning of methane gas in oxygen is: CH 4 + 2 O 2 → CO 2 + 2 H 2 O Subscript Coefficient Shows # of atoms Shows # of molecules
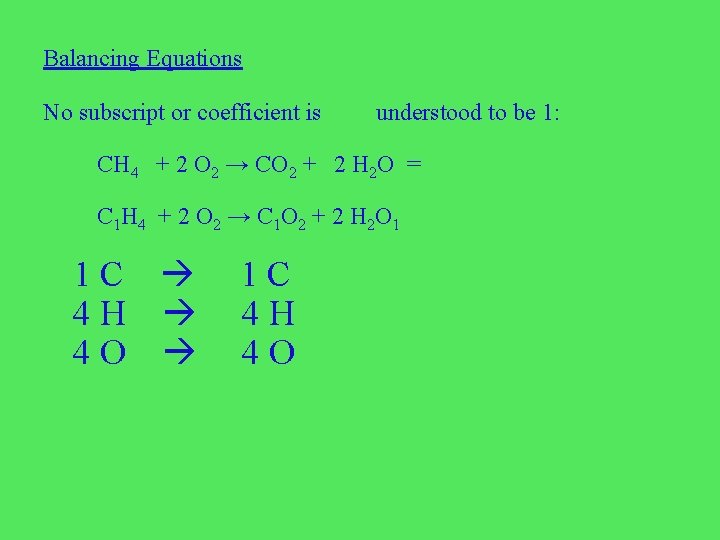
Balancing Equations No subscript or coefficient is understood to be 1: CH 4 + 2 O 2 → CO 2 + 2 H 2 O = C 1 H 4 + 2 O 2 → C 1 O 2 + 2 H 2 O 1 1 C 4 H 4 O 1 C 4 H 4 O
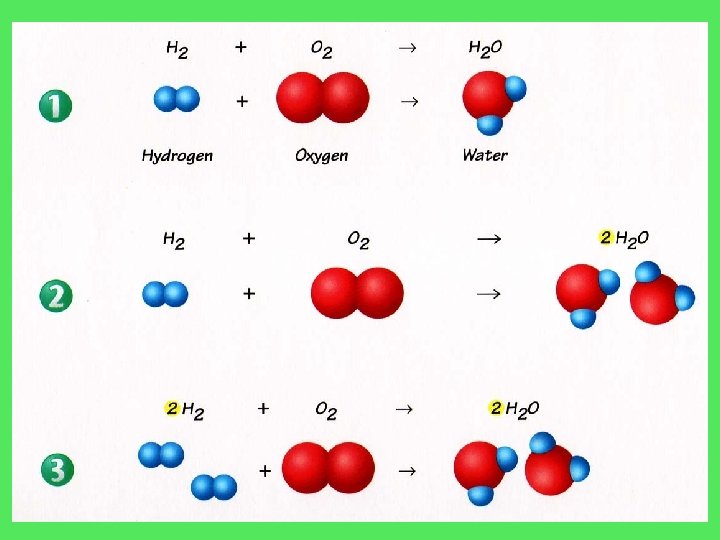
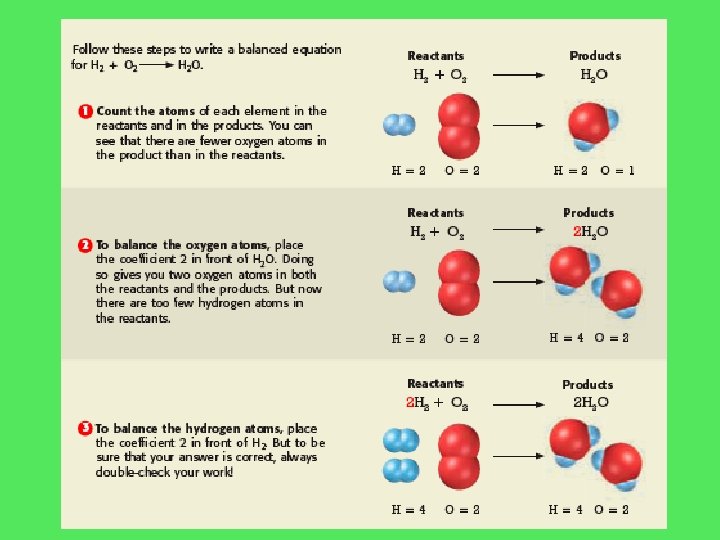
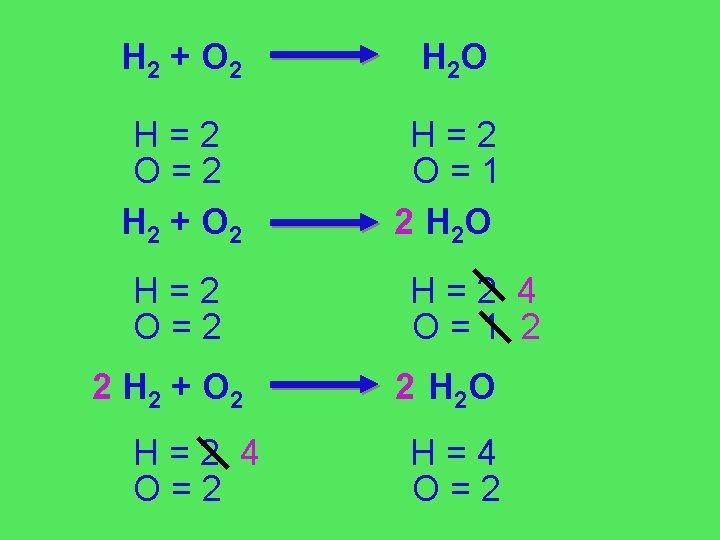
H 2 + O 2 H 2 O H=2 O=2 H 2 + O 2 H=2 O=1 2 H 2 O H=2 O=2 2 H 2 + O 2 H=2 4 O=1 2 2 H 2 O H=4 O=2
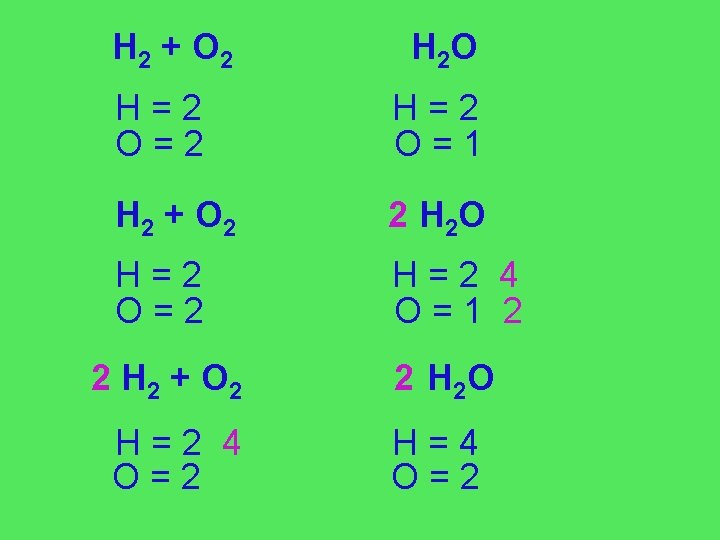
H 2 + O 2 H 2 O H=2 O=2 H=2 O=1 H 2 + O 2 2 H 2 O H=2 O=2 H=2 4 O=1 2 2 H 2 + O 2 2 H 2 O H=2 4 O=2 H=4 O=2
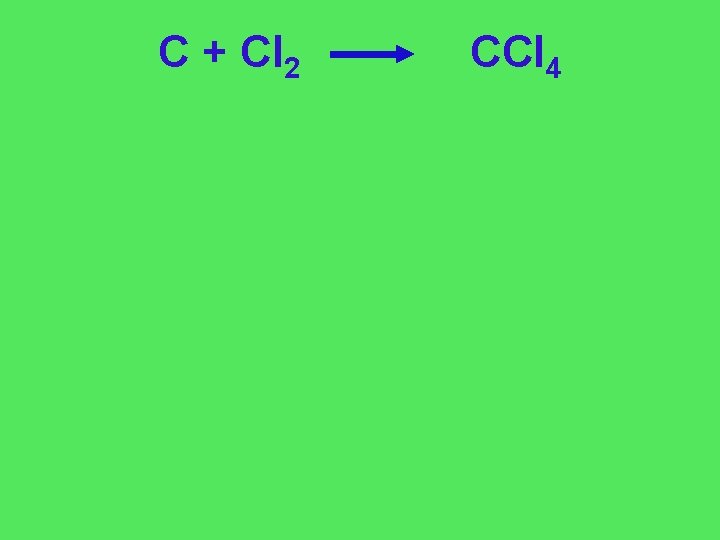
C + Cl 2 CCl 4
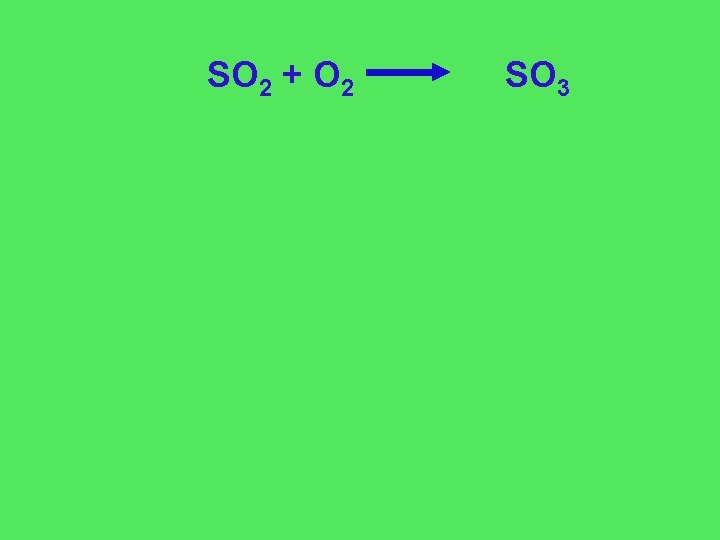
SO 2 + O 2 SO 3
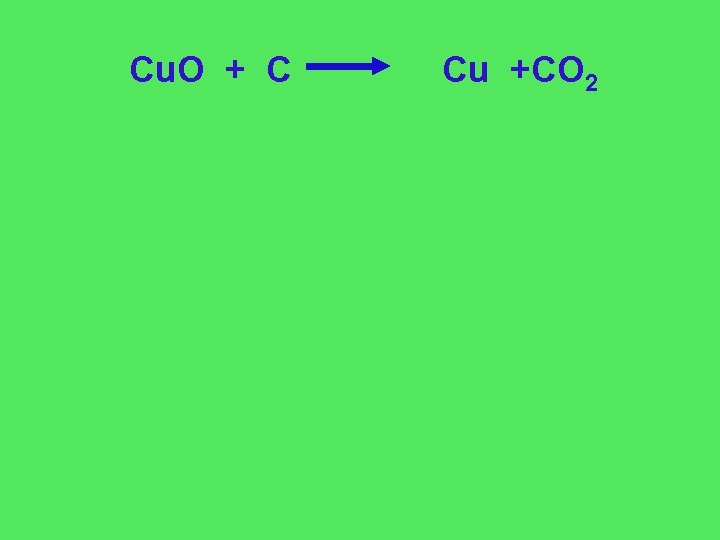
Cu. O + C Cu +CO 2
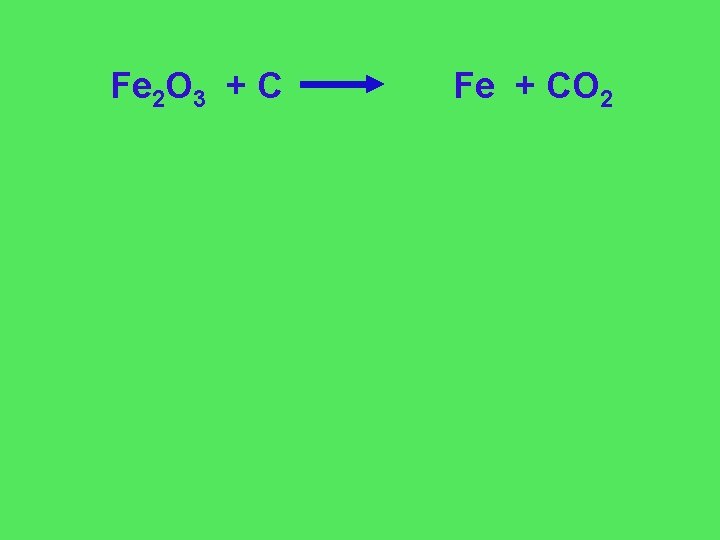
Fe 2 O 3 + C Fe + CO 2
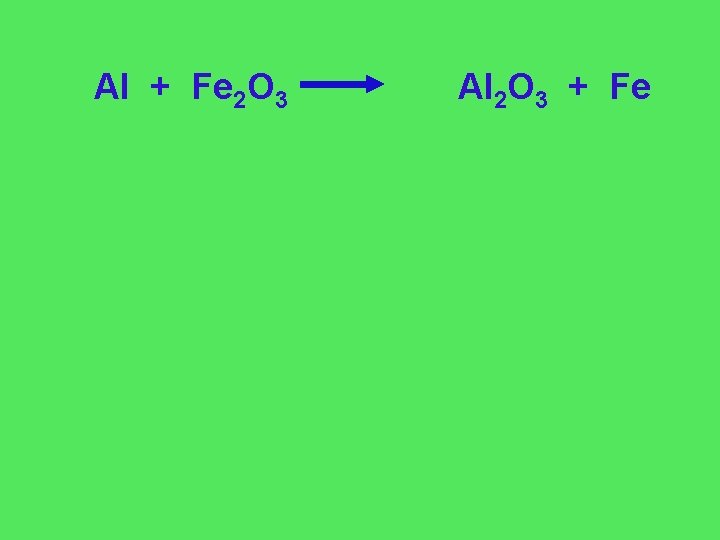
Al + Fe 2 O 3 Al 2 O 3 + Fe
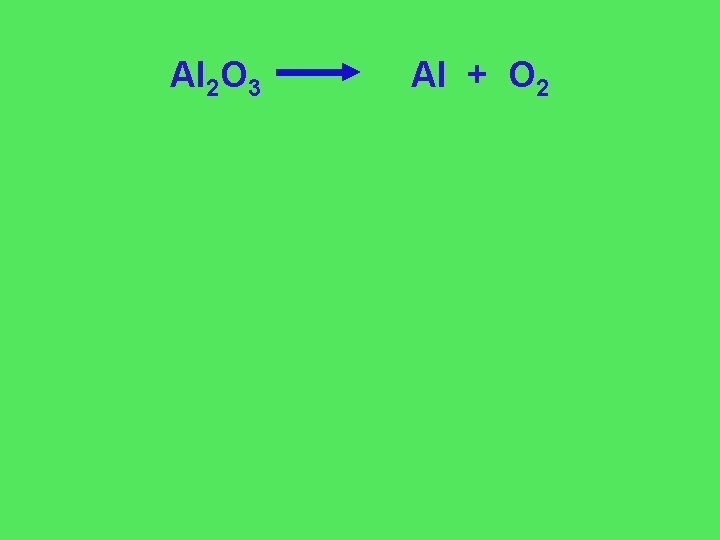
Al 2 O 3 Al + O 2
- Slides: 15