Balancing and Chemical Reactions Why do we have

Balancing and Chemical Reactions

Why do we have to Balance equations? • Hint: Think about Law of Conservation of Mass! • The # of atoms of each element must be equal on both sides of the equation.

Parts of a Chemical Equation • Parts of a Chemical Formula Chemical formula is a math formula for science! Label the different parts? 2 Na + Cl 2 2 Na. Cl Reactants=Left side of equation “and” Products=Right side of Equation “yields, produces, forms” Same as an = sign Coefficient-used to balance a chemical equation Subscript-determines how many atoms of an element are needed for the compound

Vocabulary Continued 3) Catalyst - substance that changes the rate of a chemical reaction but remains unchanged throughout the reaction

Symbols (s) (l) (g) (aq) Pt Yields: indicates result of reaction Reversible reaction Indicates a precipitate (solid) Indicates a gaseous product Reactant or product in a solid state Reactant or product in a liquid state Reactant or product in a gaseous state Reactant or product in a aqueous state Reactants are heated Presence of a catalyst

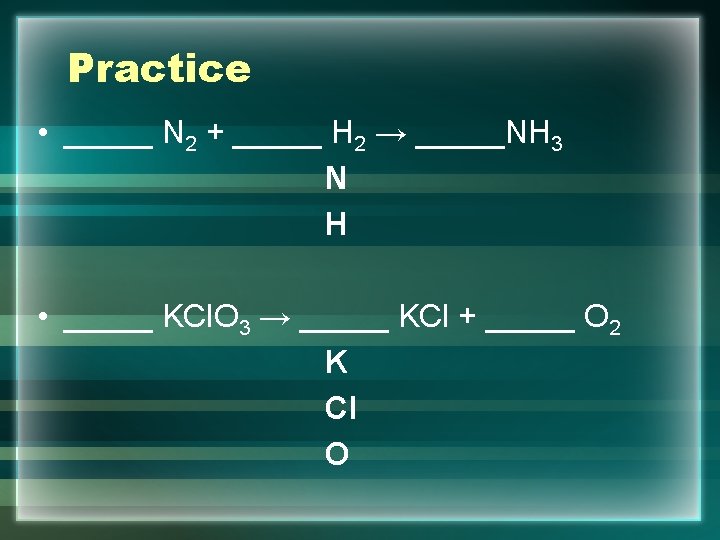
Steps to Balance an Equation • Step 1: List all the elements in the equation underneath the center of the equation • Step 2: Count how many of each element is on the reactant side then the product side • Step 3: Is this balanced or unbalanced • Step 4: balance the equation if necessary by adding coefficients
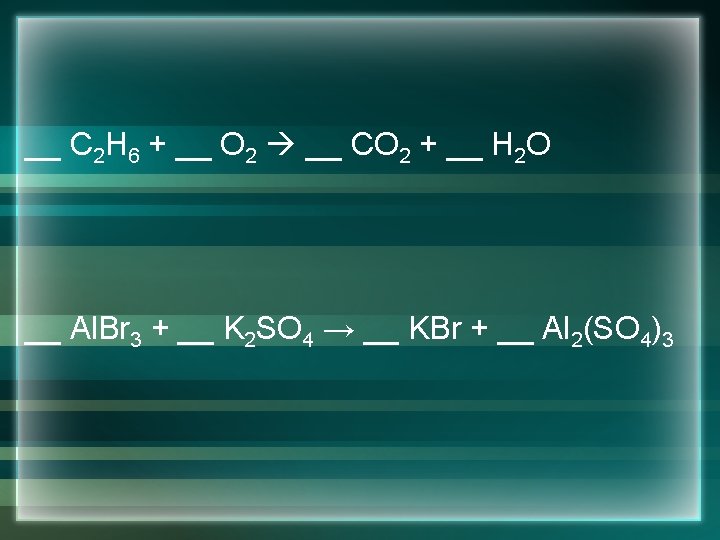
__ C 2 H 6 + __ O 2 __ CO 2 + __ H 2 O __ Al. Br 3 + __ K 2 SO 4 → __ KBr + __ Al 2(SO 4)3

Types of Reactions Notes

Reduction-Oxidation Reactions (REDOX) • Reduction-Oxidation Reactions: any chemical reaction where elements undergo changes in oxidation number (charges) • How can I tell if this is REDOX or not? – Elements that are NOT in a compound will have a charge of ZERO. – Elements in compounds will have a CHARGE

REDOX Vocabulary 1) Reduced: the oxidation state (charge) of the element decreases from the reactants to the products 2) Oxidized: the oxidation state (charge) of the element increases from the reactants to products
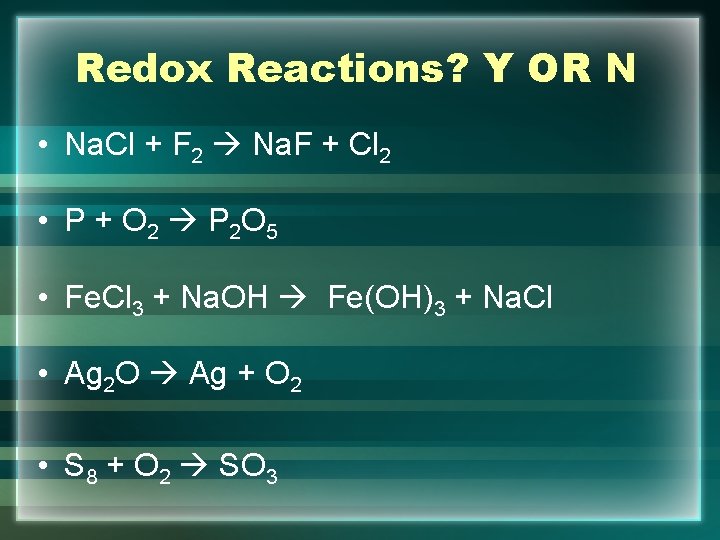
Redox Reactions? Y OR N • Na. Cl + F 2 Na. F + Cl 2 • P + O 2 P 2 O 5 • Fe. Cl 3 + Na. OH Fe(OH)3 + Na. Cl • Ag 2 O Ag + O 2 • S 8 + O 2 SO 3
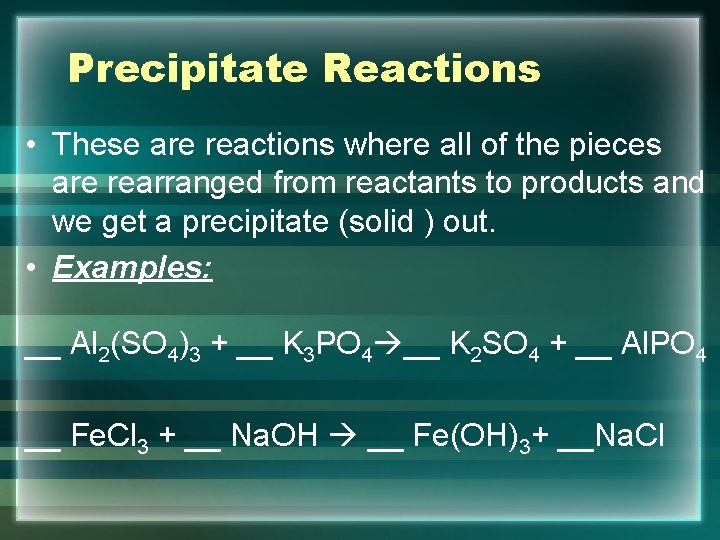
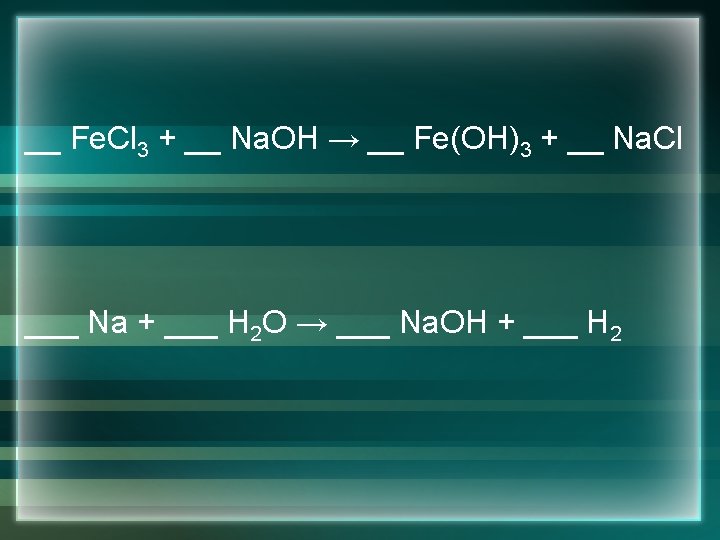
Precipitate Reactions • These are reactions where all of the pieces are rearranged from reactants to products and we get a precipitate (solid ) out. • Examples: __ Al 2(SO 4)3 + __ K 3 PO 4 __ K 2 SO 4 + __ Al. PO 4 __ Fe. Cl 3 + __ Na. OH __ Fe(OH)3+ __Na. Cl

Precipitate Vocabulary 1) Precipitate: solid produced within a solution as a result of a chemical reaction 2) Aqueous solution: a solution where the solvent is water
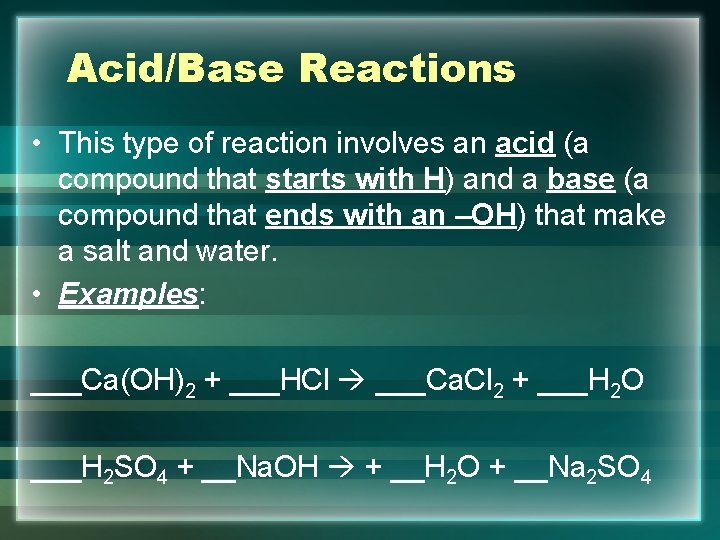
Acid/Base Reactions • This type of reaction involves an acid (a compound that starts with H) and a base (a compound that ends with an –OH) that make a salt and water. • Examples: ___Ca(OH)2 + ___HCl ___Ca. Cl 2 + ___H 2 O ___H 2 SO 4 + __Na. OH + __H 2 O + __Na 2 SO 4
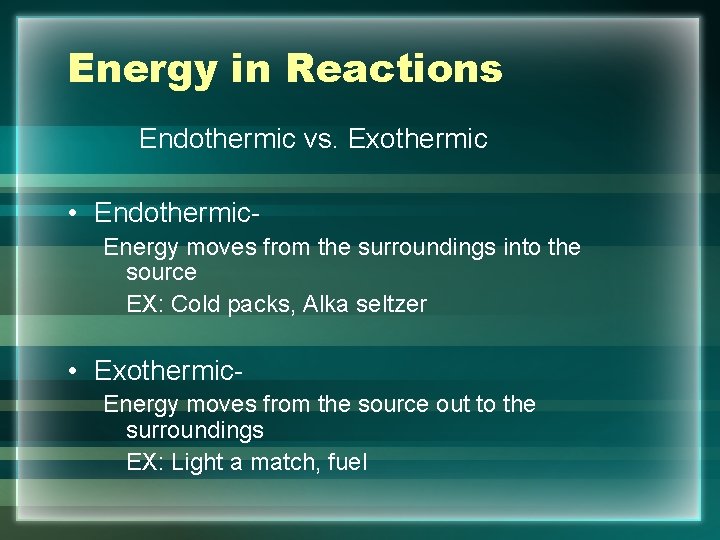
Energy in Reactions Endothermic vs. Exothermic • Endothermic. Energy moves from the surroundings into the source EX: Cold packs, Alka seltzer • Exothermic. Energy moves from the source out to the surroundings EX: Light a match, fuel

Types of Reactions Notes

Why do chemicals react? • You already know!!!!! – To fill up their outer shells… • when we create chemical bonds we have a chemical reaction!! • Types: – Ionic – Covalent – Metallic
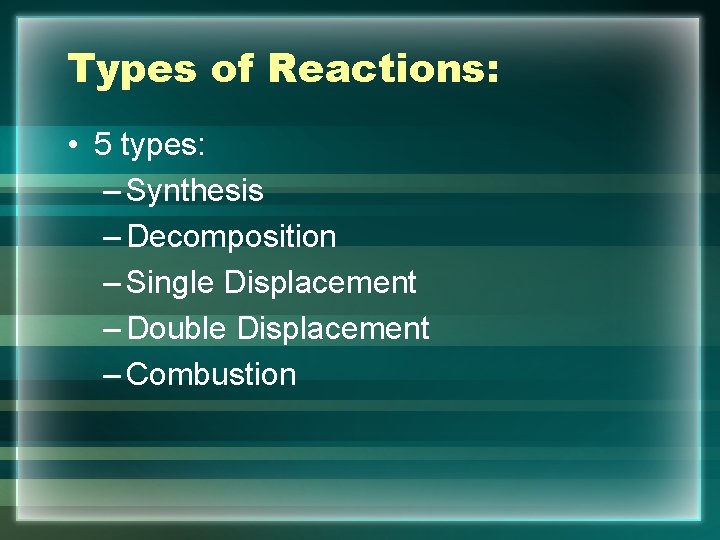
Types of Reactions: • 5 types: – Synthesis – Decomposition – Single Displacement – Double Displacement – Combustion
Synthesis • Two or more elements combine to form 1 chemical compound – EX: 2 Mg + O 2 2 Mg. O – Analogy: One girl + One Boy = One couple • Generic equation: A + B AB • Another example: Water Formation
Decomposition • When a compound breaks into 2 elements or simpler compounds-“break down” – 2 Na. Cl = 2 Na + Cl 2 – Analogy: One couple= One boy + one girl (Divorce) • Generic equation: AB A + B • Another example: ”Electrolysis” Decomposition
Single Displacement • A single uncombined element replaces another in a compound. Two reactants yield two products. Analogy: A “playa girl” has a boyfriend but likes a cuter boy instead SD Animation • Generic Example: AB + C A + BC Example: Lets visualize it!
Double Displacement • When one element of each compound changes places with the other element – Analogy: Two couples that switch partners • Generic Example: AB + CD AC + BD • Example: Pb. Cl 2 + Li 2 SO 4 Pb. SO 4 + 2 Li. Cl DD RX

Combustion Reaction • What do we know as combustion? – FIRE!!!! – Requires a fuel and oxygen and produces CO 2 and H 2 O – Releases so much energy a flame is released • Generic example: C(x)H(x)+O 2→H 2 O(g)+CO 2(g) Combustion Reaction

Balancing Chemical Equations • Why do we need to balance? – Law of Conservation of Mass-mass can not be created or destroyed, it is only changed to a different form • Everything on the left has to equal everything on the right!!! • Just like math… 3 + 0 = 5 WRONG!!! 3 + 2 = 5 RIGHT!!!
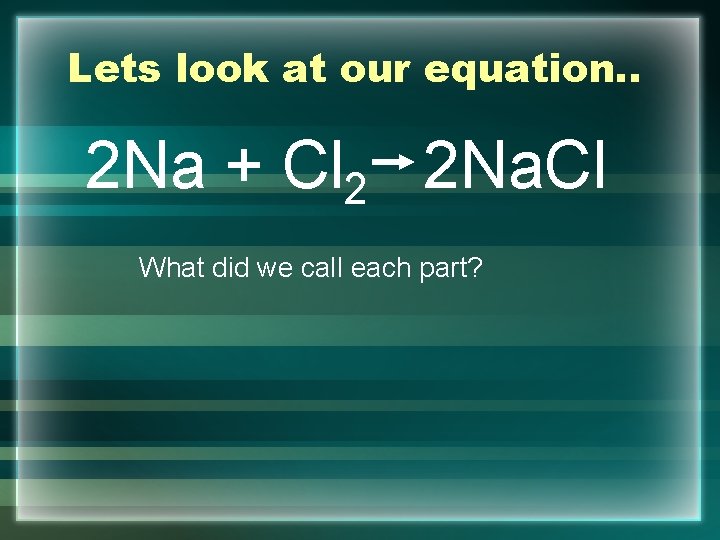
Lets look at our equation. . 2 Na + Cl 2 2 Na. Cl What did we call each part?

Word Equations Must know the Diatomic Molecules: Hydrogen – H 2 Nitrogen – N 2 Oxygen – O 2 Fluorine – F 2 Chlorine – Cl 2 Bromine – Br 2 Iodine – I 2

Word Equations Practice • As I quoted “You cannot forget how to write formulas!” Practice: 1) iron (III) chloride and ammonium hydroxide produce iron (III) hydroxide and ammonium chloride

Word Equations Practice 2) potassium and chlorine combine to form potassium chloride 3) ammonium nitrate yields dinitrogen monoxide and water

4) dicarbon dihydride and oxygen produce carbon dioxide and water 5) aluminum bromide and chlorine react to form aluminum chloride and bromine
- Slides: 31