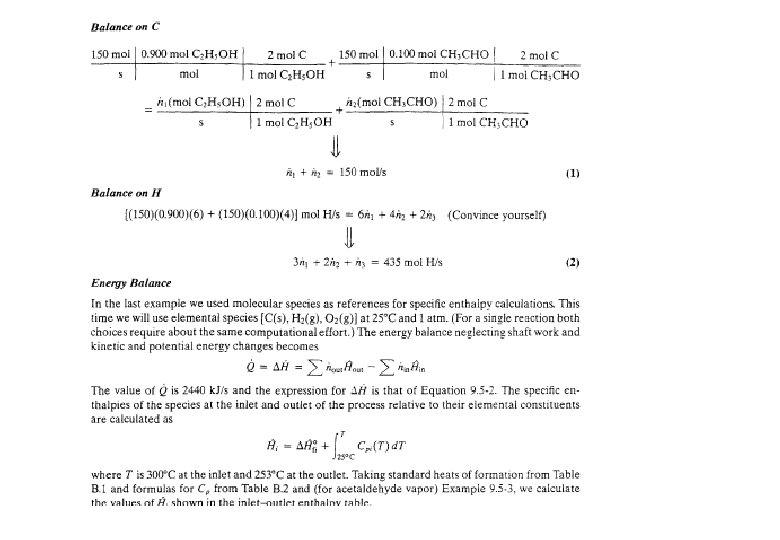
Balances on Reactive Processes II Shamsuzzoha Ph D

Balances on Reactive Processes II Shamsuzzoha, Ph. D Department of Chemical Engineering, KFUPM
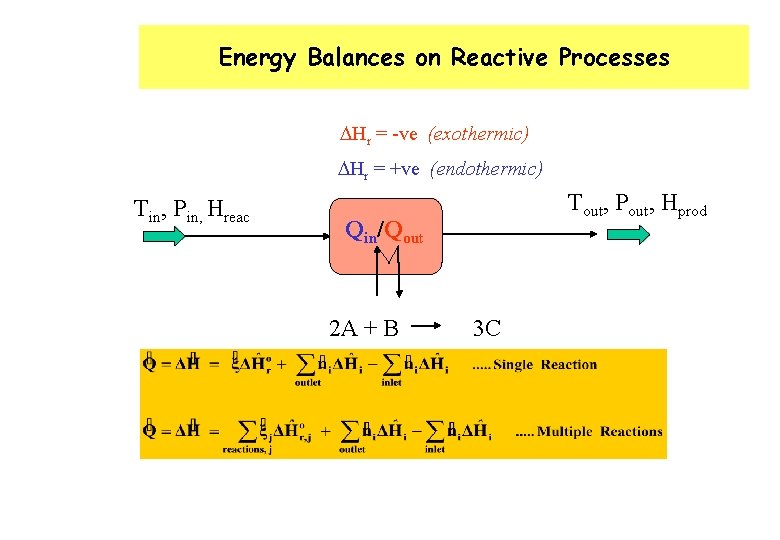
Energy Balances on Reactive Processes DHr = -ve (exothermic) DHr = +ve (endothermic) Tin, Pin, Hreac Tout, Pout, Hprod Qin/Qout 2 A + B 3 C
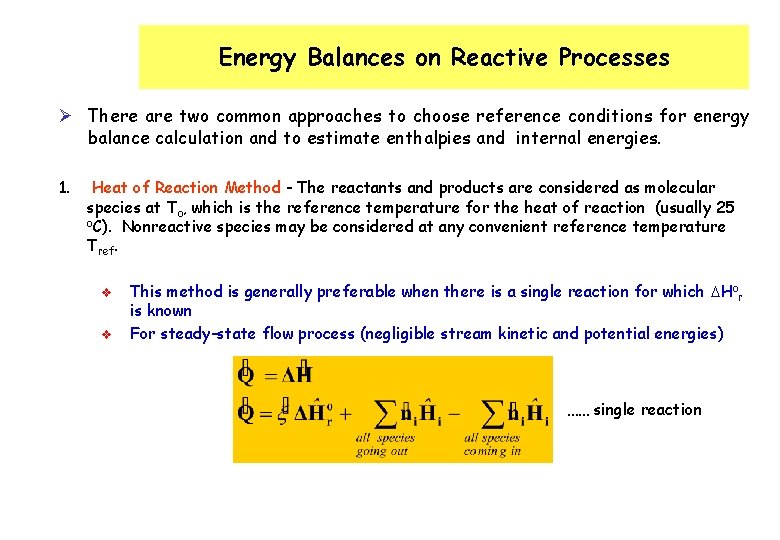
Energy Balances on Reactive Processes Ø There are two common approaches to choose reference conditions for energy balance calculation and to estimate enthalpies and internal energies. 1. Heat of Reaction Method - The reactants and products are considered as molecular species at To, which is the reference temperature for the heat of reaction (usually 25 o. C). Nonreactive species may be considered at any convenient reference temperature Tref. v v This method is generally preferable when there is a single reaction for which DHor is known For steady-state flow process (negligible stream kinetic and potential energies) …… single reaction
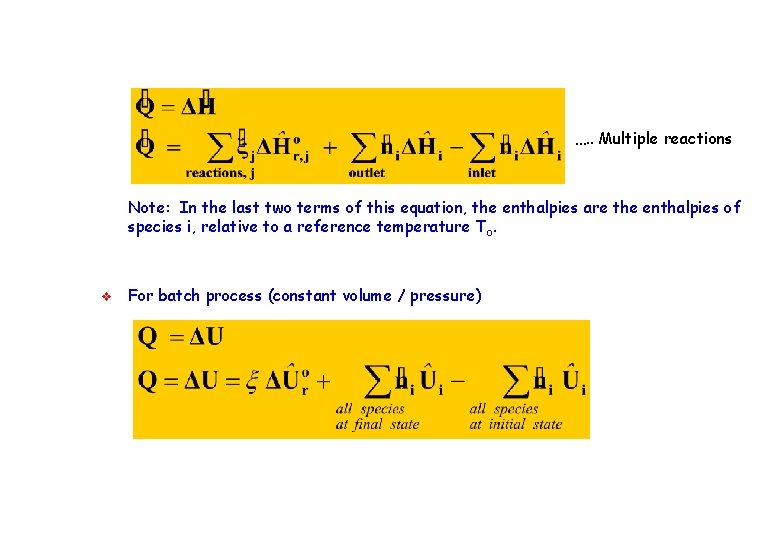
…. . Multiple reactions Note: In the last two terms of this equation, the enthalpies are the enthalpies of species i, relative to a reference temperature T o. v For batch process (constant volume / pressure)
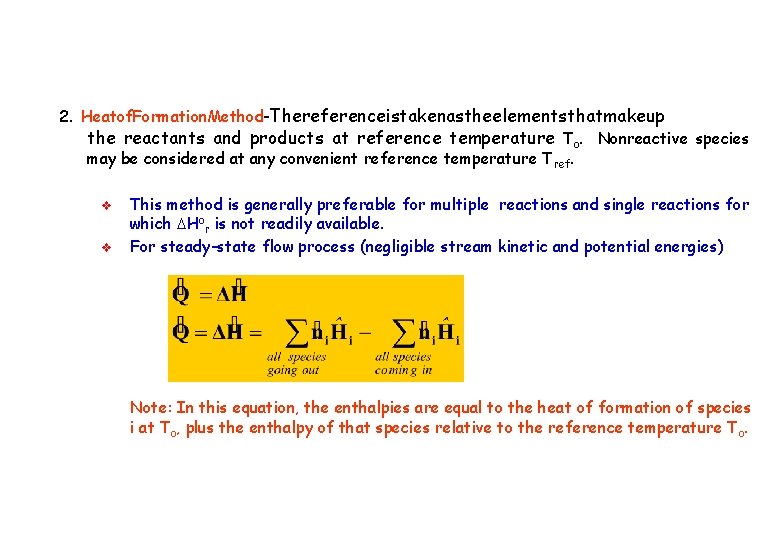
2. Heatof. Formation. Method-Thereferenceistakenastheelementsthatmakeup the reactants and products at reference temperature To. Nonreactive species may be considered at any convenient reference temperature T ref. v v This method is generally preferable for multiple reactions and single reactions for which DHor is not readily available. For steady-state flow process (negligible stream kinetic and potential energies) Note: In this equation, the enthalpies are equal to the heat of formation of species i at To, plus the enthalpy of that species relative to the reference temperature T o.
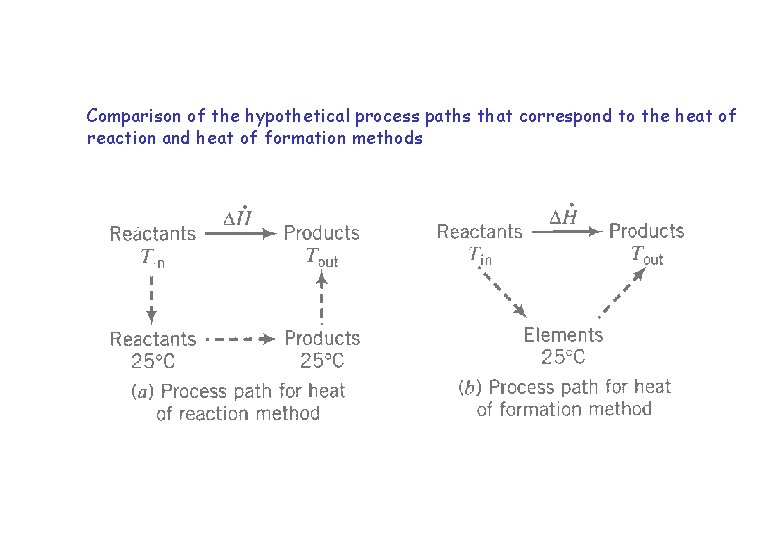
Comparison of the hypothetical process paths that correspond to the heat of reaction and heat of formation methods
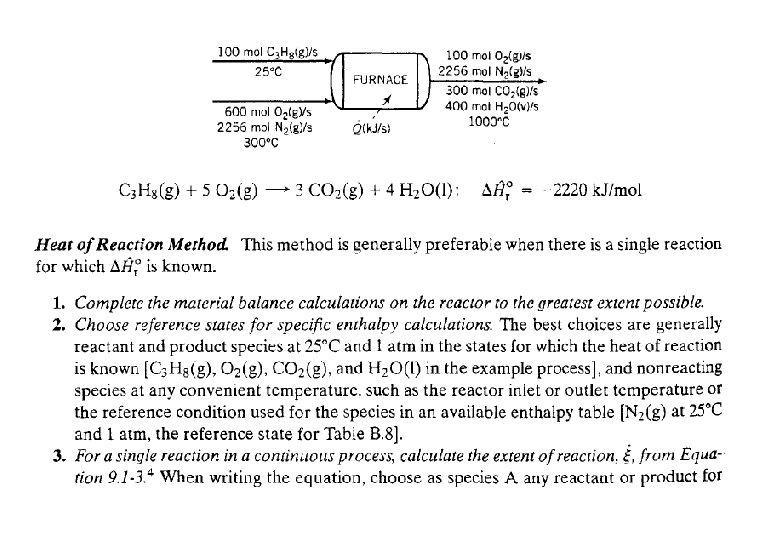
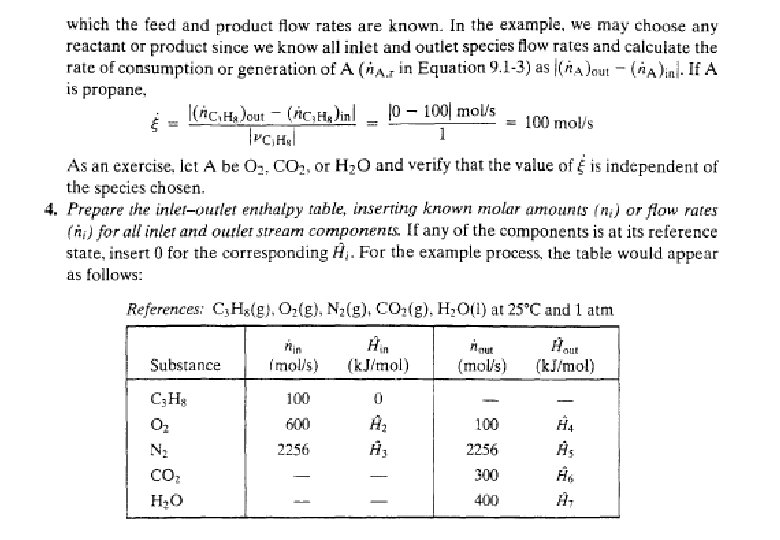
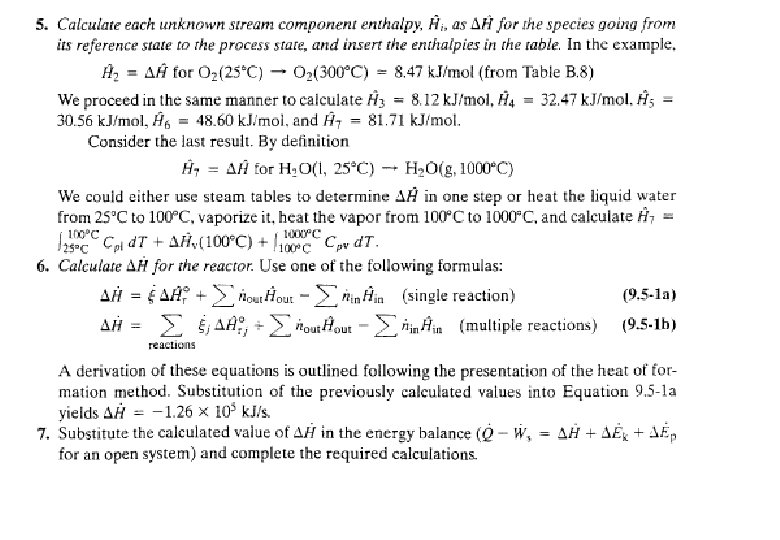
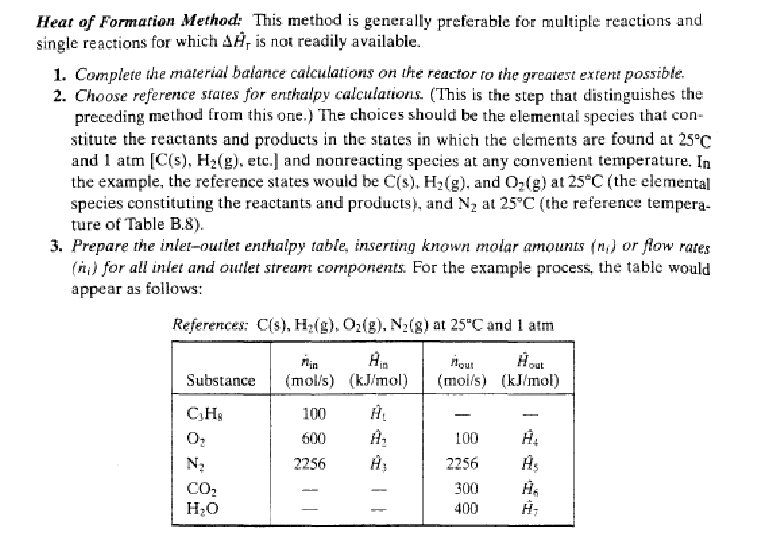
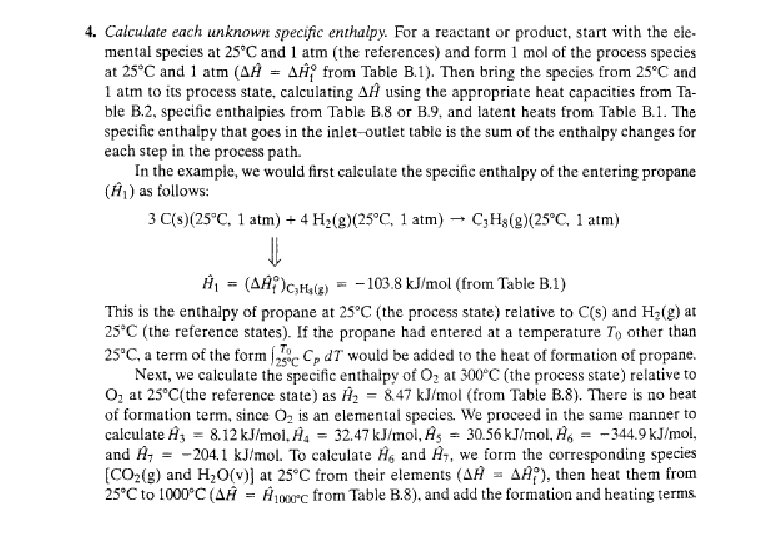
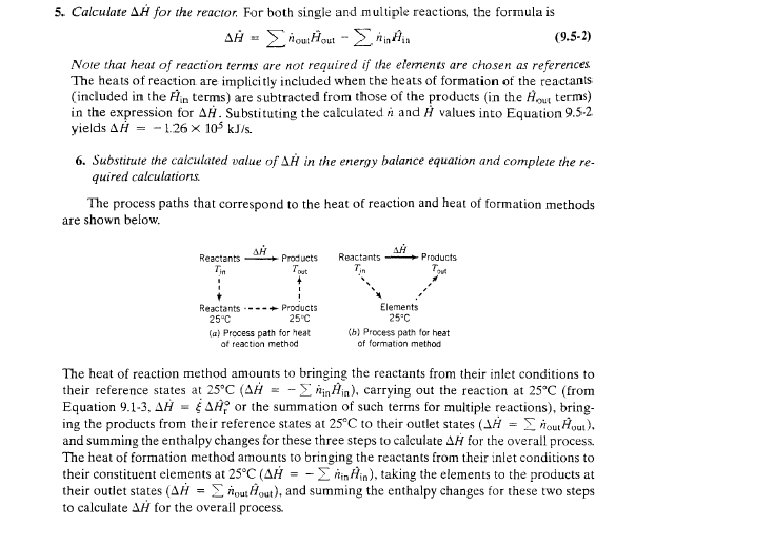
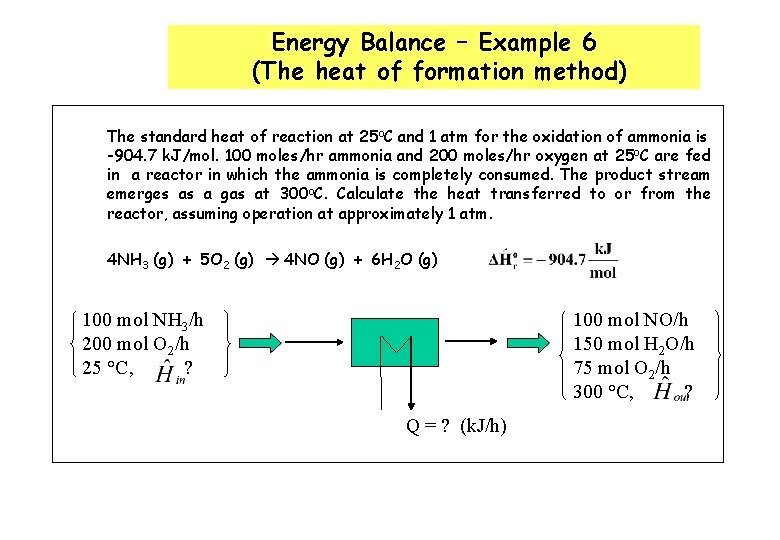
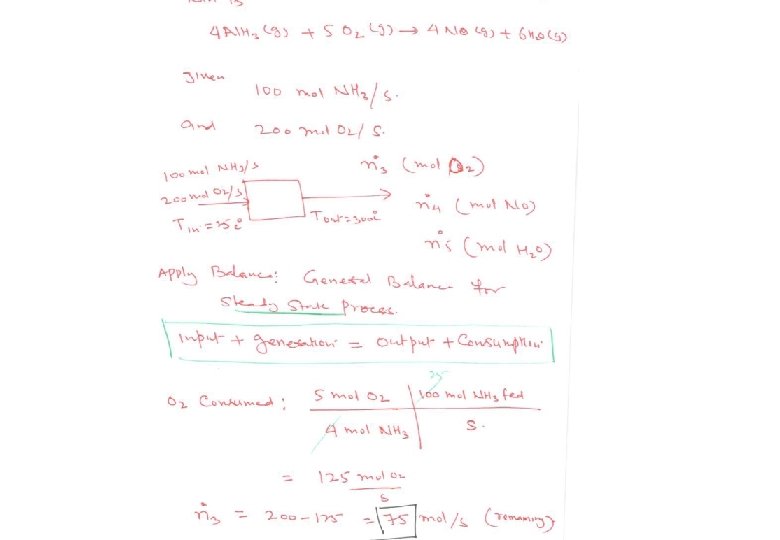
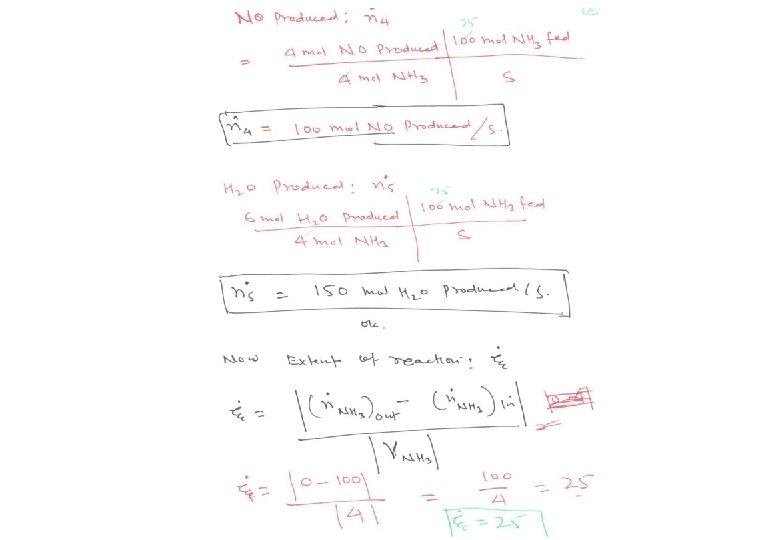
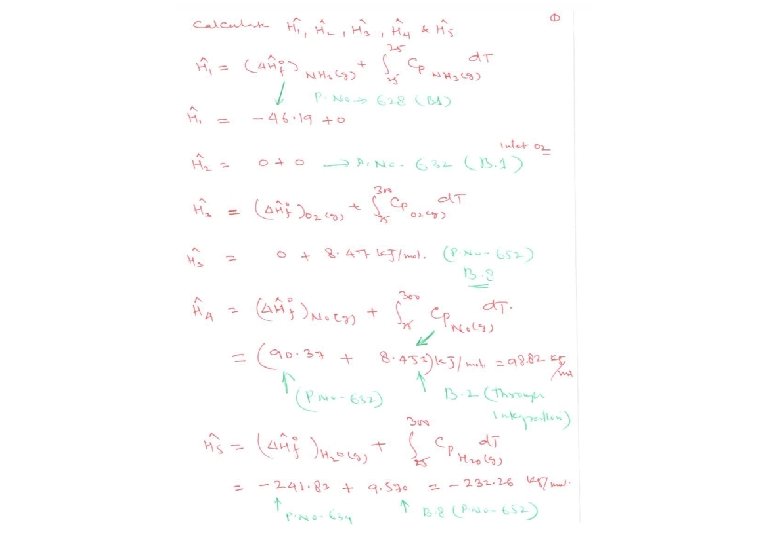
Energy Balance – Example 6 (The heat of formation method) The standard heat of reaction at 25 o. C and 1 atm for the oxidation of ammonia is -904. 7 k. J/mol. 100 moles/hr ammonia and 200 moles/hr oxygen at 25 o. C are fed in a reactor in which the ammonia is completely consumed. The product stream emerges as a gas at 300 o. C. Calculate the heat transferred to or from the reactor, assuming operation at approximately 1 atm. 4 NH 3 (g) + 5 O 2 (g) 4 NO (g) + 6 H 2 O (g) 100 mol NH 3/h 200 mol O 2/h 25 °C, ? 100 mol NO/h 150 mol H 2 O/h 75 mol O 2/h 300 °C, ? Q = ? (k. J/h)
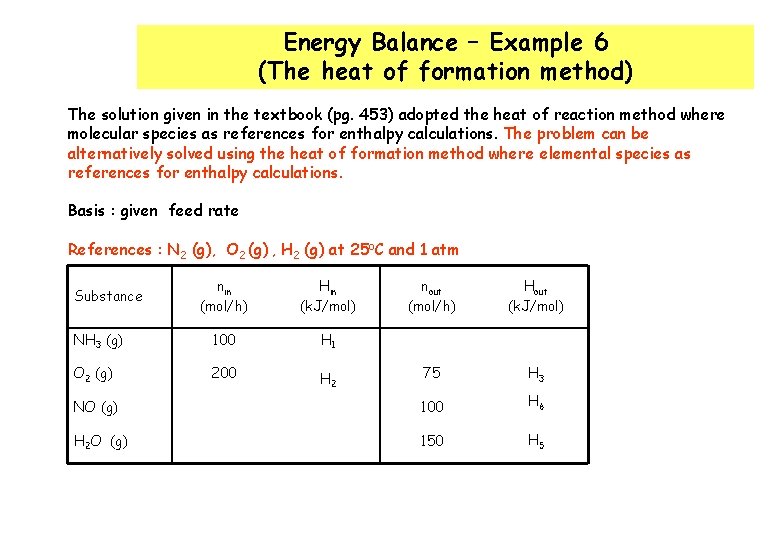
Energy Balance – Example 6 (The heat of formation method) The solution given in the textbook (pg. 453) adopted the heat of reaction method where molecular species as references for enthalpy calculations. The problem can be alternatively solved using the heat of formation method where elemental species as references for enthalpy calculations. Basis : given feed rate References : N 2 (g), O 2 (g) , H 2 (g) at 25 o. C and 1 atm nin (mol/h) Hin (k. J/mol) nout (mol/h) Hout (k. J/mol) NH 3 (g) 100 H 1 O 2 (g) 200 H 2 75 H 3 NO (g) 100 H 6 H 2 O (g) 150 H 5 Substance
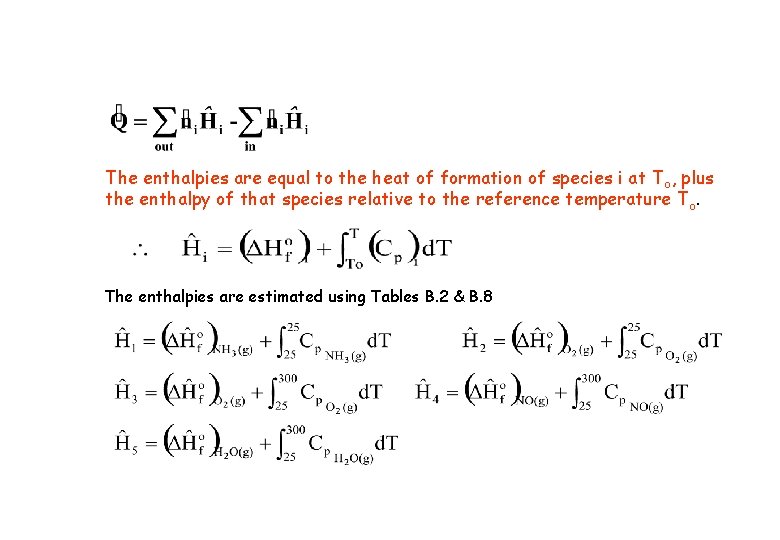
The enthalpies are equal to the heat of formation of species i at T o, plus the enthalpy of that species relative to the reference temperature T o. The enthalpies are estimated using Tables B. 2 & B. 8

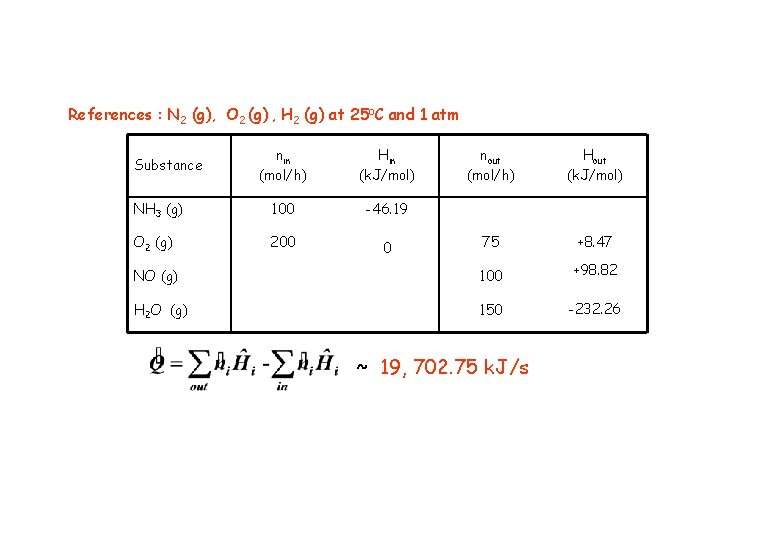
References : N 2 (g), O 2 (g) , H 2 (g) at 25 o. C and 1 atm nin (mol/h) Hin (k. J/mol) nout (mol/h) Hout (k. J/mol) NH 3 (g) 100 -46. 19 O 2 (g) 200 0 75 +8. 47 NO (g) 100 +98. 82 H 2 O (g) 150 -232. 26 Substance ~ 19, 702. 75 k. J/s
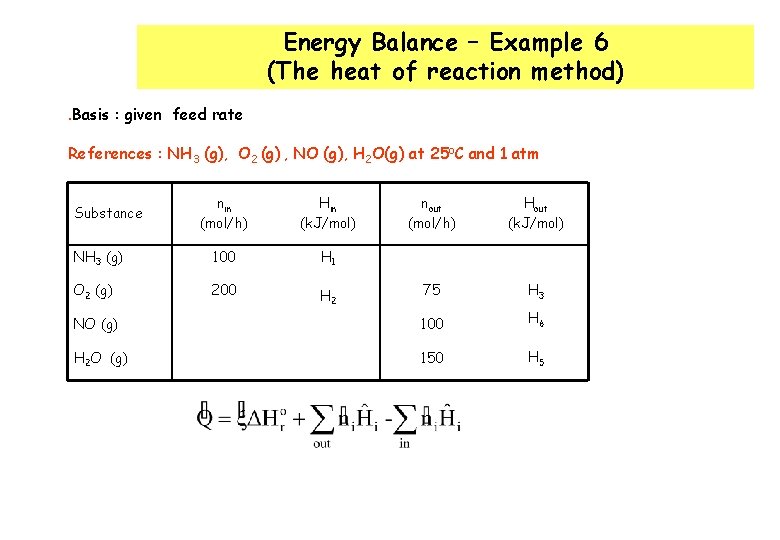
Energy Balance – Example 6 (The heat of reaction method). Basis : given feed rate References : NH 3 (g), O 2 (g) , NO (g), H 2 O(g) at 25 o. C and 1 atm nin (mol/h) Hin (k. J/mol) nout (mol/h) Hout (k. J/mol) NH 3 (g) 100 H 1 O 2 (g) 200 H 2 75 H 3 NO (g) 100 H 6 H 2 O (g) 150 H 5 Substance
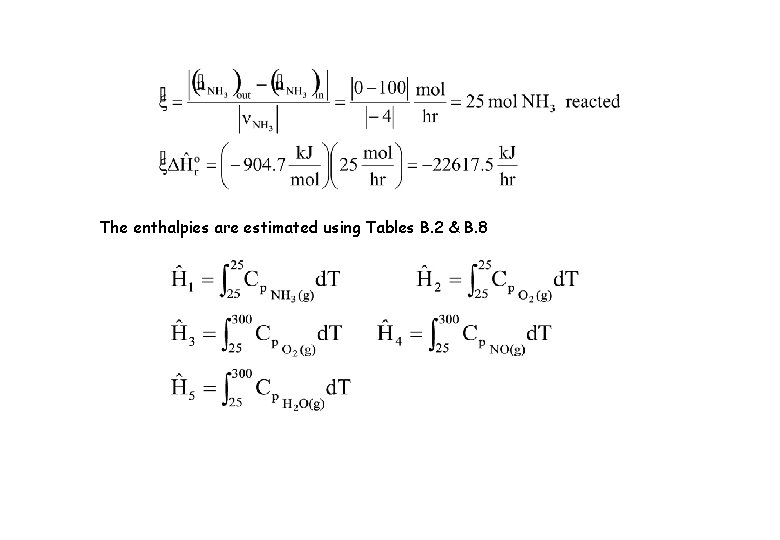
The enthalpies are estimated using Tables B. 2 & B. 8

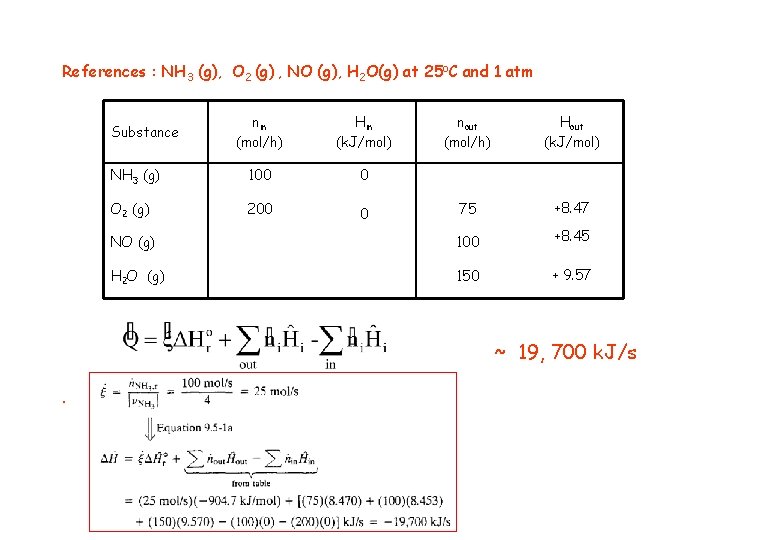
References : NH 3 (g), O 2 (g) , NO (g), H 2 O(g) at 25 o. C and 1 atm nin (mol/h) Hin (k. J/mol) nout (mol/h) Hout (k. J/mol) NH 3 (g) 100 0 O 2 (g) 200 0 75 +8. 47 NO (g) 100 +8. 45 H 2 O (g) 150 + 9. 57 Substance ~ 19, 700 k. J/s.
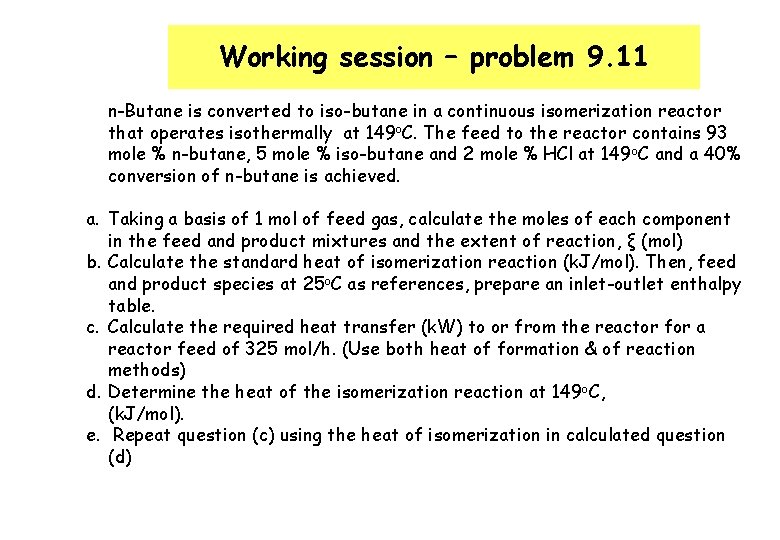
Working session – problem 9. 11 n-Butane is converted to iso-butane in a continuous isomerization reactor that operates isothermally at 149 o. C. The feed to the reactor contains 93 mole % n-butane, 5 mole % iso-butane and 2 mole % HCl at 149 o. C and a 40% conversion of n-butane is achieved. a. Taking a basis of 1 mol of feed gas, calculate the moles of each component in the feed and product mixtures and the extent of reaction, ξ (mol) b. Calculate the standard heat of isomerization reaction (k. J/mol). Then, feed and product species at 25 o. C as references, prepare an inlet-outlet enthalpy table. c. Calculate the required heat transfer (k. W) to or from the reactor for a reactor feed of 325 mol/h. (Use both heat of formation & of reaction methods) d. Determine the heat of the isomerization reaction at 149 o. C, (k. J/mol). e. Repeat question (c) using the heat of isomerization in calculated question (d)
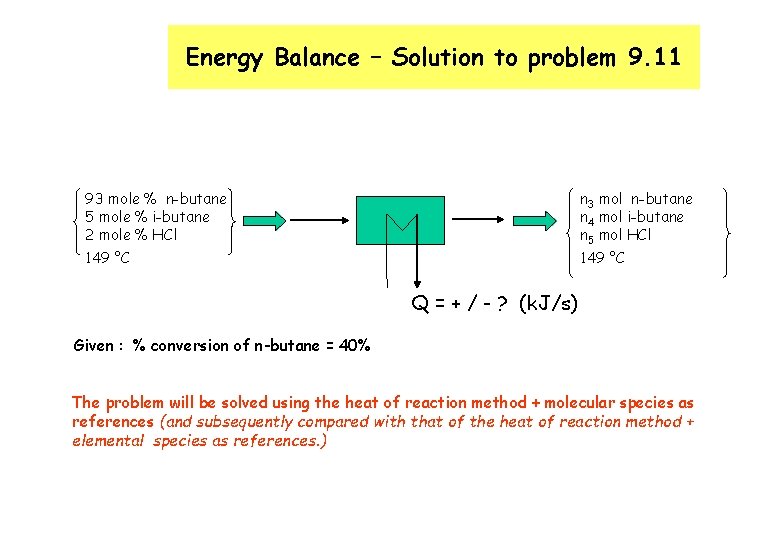
Energy Balance – Solution to problem 9. 11 n 3 mol n-butane n 4 mol i-butane n 5 mol HCl 93 mole % n-butane 5 mole % i-butane 2 mole % HCl 149 °C Q = + / - ? (k. J/s) Given : % conversion of n-butane = 40% The problem will be solved using the heat of reaction method + molecular species as references (and subsequently compared with that of the heat of reaction method + elemental species as references. )
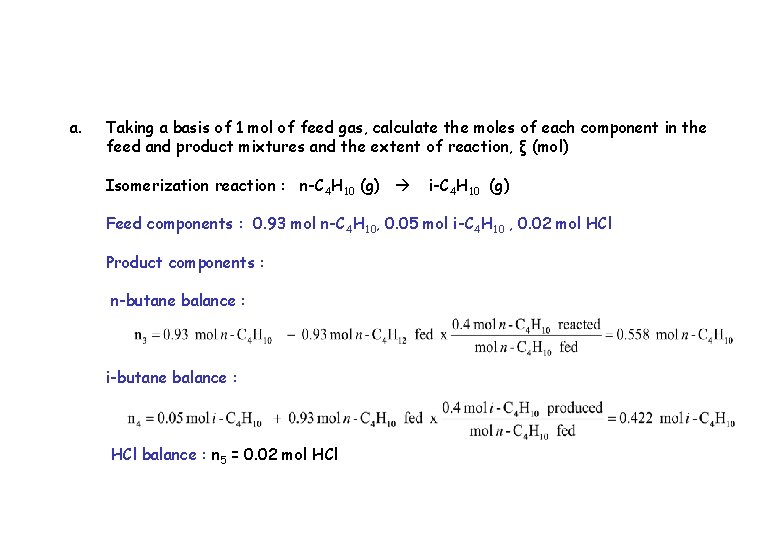
a. Taking a basis of 1 mol of feed gas, calculate the moles of each component in the feed and product mixtures and the extent of reaction, ξ (mol) Isomerization reaction : n-C 4 H 10 (g) i-C 4 H 10 (g) Feed components : 0. 93 mol n-C 4 H 10, 0. 05 mol i-C 4 H 10 , 0. 02 mol HCl Product components : n-butane balance : i-butane balance : HCl balance : n 5 = 0. 02 mol HCl
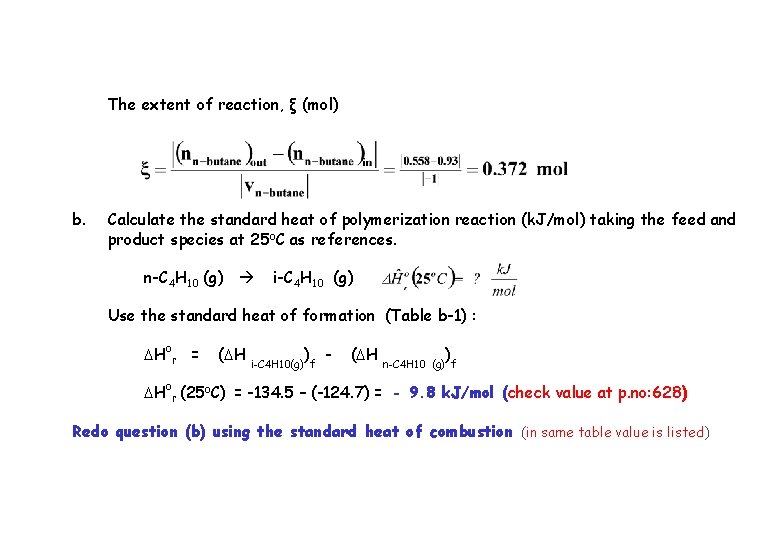
The extent of reaction, ξ (mol) b. Calculate the standard heat of polymerization reaction (k. J/mol) taking the feed and product species at 25 o. C as references. n-C 4 H 10 (g) i-C 4 H 10 (g) Use the standard heat of formation (Table b-1) : DHor = (DH i-C 4 H 10(g))f - (DH n-C 4 H 10 ) (g) f DHor (25 o. C) = -134. 5 – (-124. 7) = - 9. 8 k. J/mol (check value at p. no: 628) Redo question (b) using the standard heat of combustion (in same table value is listed)
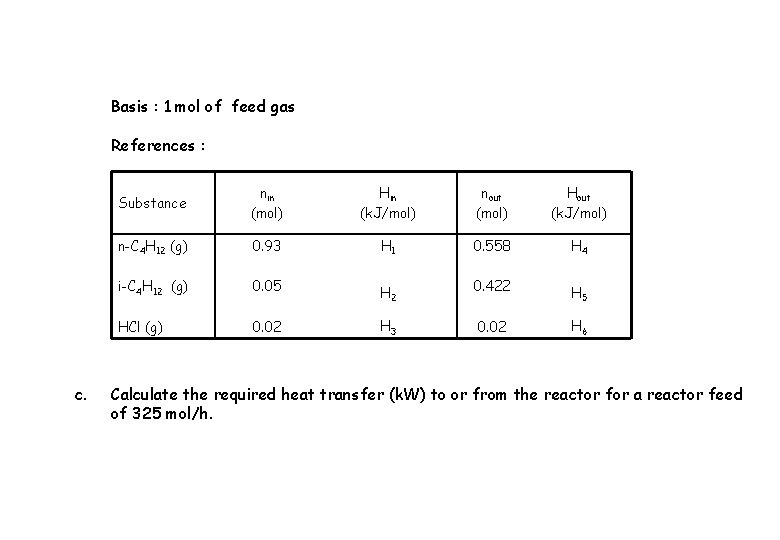
Basis : 1 mol of feed gas References : c. Substance nin (mol) Hin (k. J/mol) nout (mol) Hout (k. J/mol) n-C 4 H 12 (g) 0. 93 H 1 0. 558 H 4 i-C 4 H 12 (g) 0. 05 H 2 0. 422 H 5 HCl (g) 0. 02 H 3 0. 02 H 6 Calculate the required heat transfer (k. W) to or from the reactor for a reactor feed of 325 mol/h.
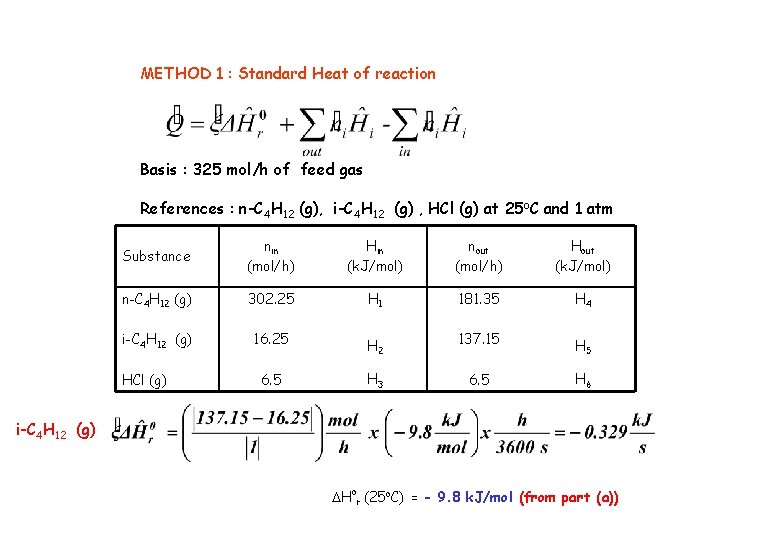
METHOD 1 : Standard Heat of reaction Basis : 325 mol/h of feed gas References : n-C 4 H 12 (g), i-C 4 H 12 (g) , HCl (g) at 25 o. C and 1 atm Substance nin (mol/h) Hin (k. J/mol) nout (mol/h) Hout (k. J/mol) n-C 4 H 12 (g) 302. 25 H 1 181. 35 H 4 i-C 4 H 12 (g) 16. 25 H 2 137. 15 H 5 6. 5 H 3 6. 5 H 6 HCl (g) i-C 4 H 12 (g) DHor (25 o. C) = - 9. 8 k. J/mol (from part (a))

Note: since process is isothermal so inlet and outlet temp is same, so for H 1 and H 4 temp is same, ok! Heat of capacities are given in Table B-2, use integration and be careful about Form of equation(either gas or liq, here we have gas only -0. 329 k. J/s + [181. 35(14. 287) + 137. 15(14. 142) + 6. 5(3. 608) – 302. 25(14. 287) – 16. 25(14. 142)-6. 5(3. 608)] k. J/h x h/3600 s -0. 334 k. J/s
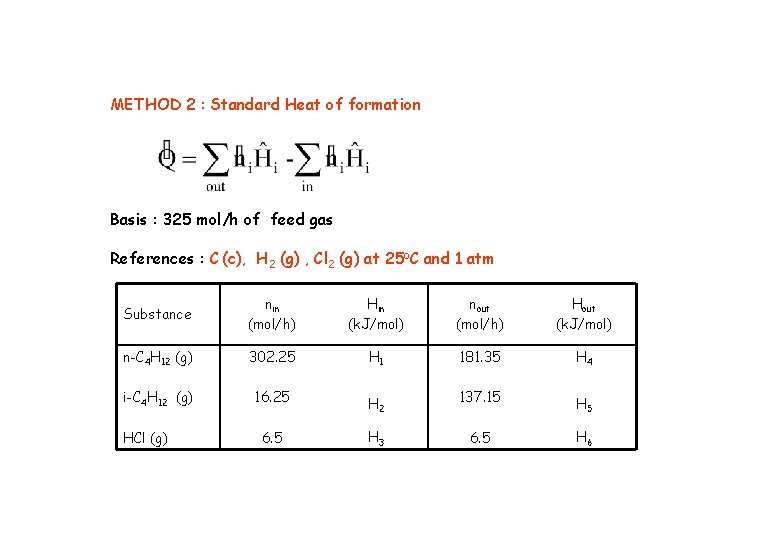
METHOD 2 : Standard Heat of formation Basis : 325 mol/h of feed gas References : C (c), H 2 (g) , Cl 2 (g) at 25 o. C and 1 atm Substance nin (mol/h) Hin (k. J/mol) nout (mol/h) Hout (k. J/mol) n-C 4 H 12 (g) 302. 25 H 1 181. 35 H 4 i-C 4 H 12 (g) 16. 25 H 2 137. 15 H 5 6. 5 H 3 6. 5 H 6 HCl (g)
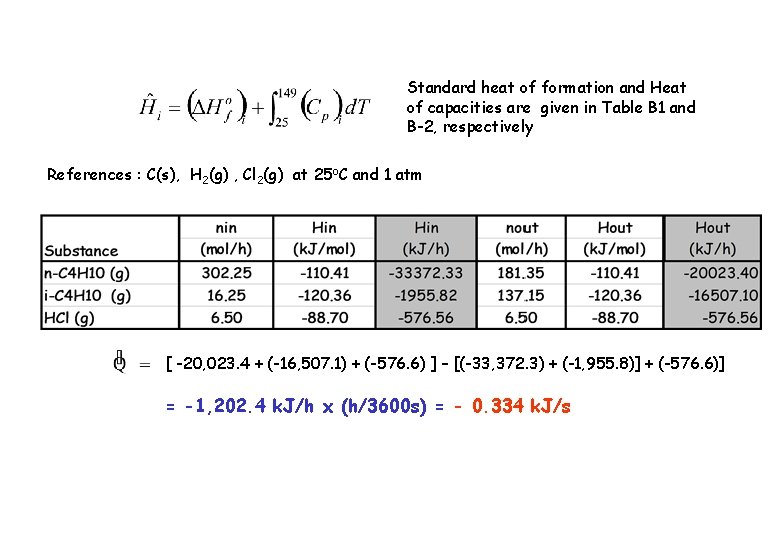
Standard heat of formation and Heat of capacities are given in Table B 1 and B-2, respectively References : C(s), H 2(g) , Cl 2(g) at 25 o. C and 1 atm [ -20, 023. 4 + (-16, 507. 1) + (-576. 6) ] – [(-33, 372. 3) + (-1, 955. 8)] + (-576. 6)] = -1, 202. 4 k. J/h x (h/3600 s) = - 0. 334 k. J/s
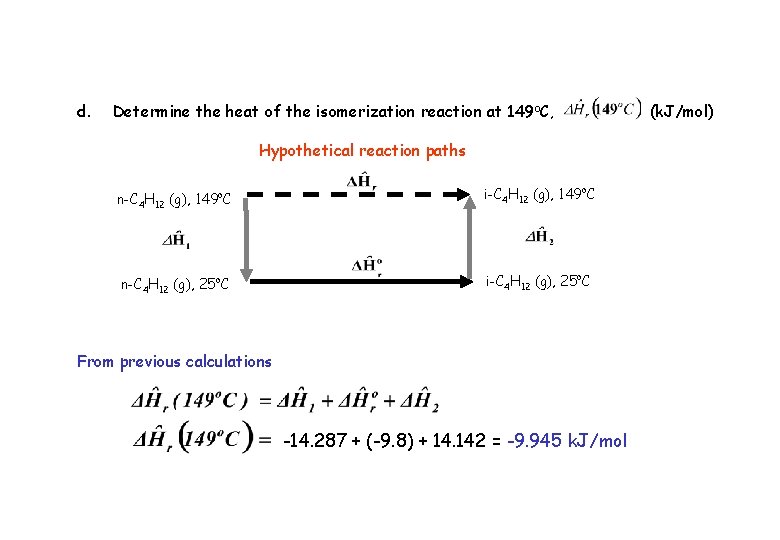
d. Determine the heat of the isomerization reaction at 149 o. C, Hypothetical reaction paths n-C 4 H 12 (g), 149 o. C i-C 4 H 12 (g), 149 o. C n-C 4 H 12 (g), 25 o. C i-C 4 H 12 (g), 25 o. C From previous calculations -14. 287 + (-9. 8) + 14. 142 = -9. 945 k. J/mol (k. J/mol)
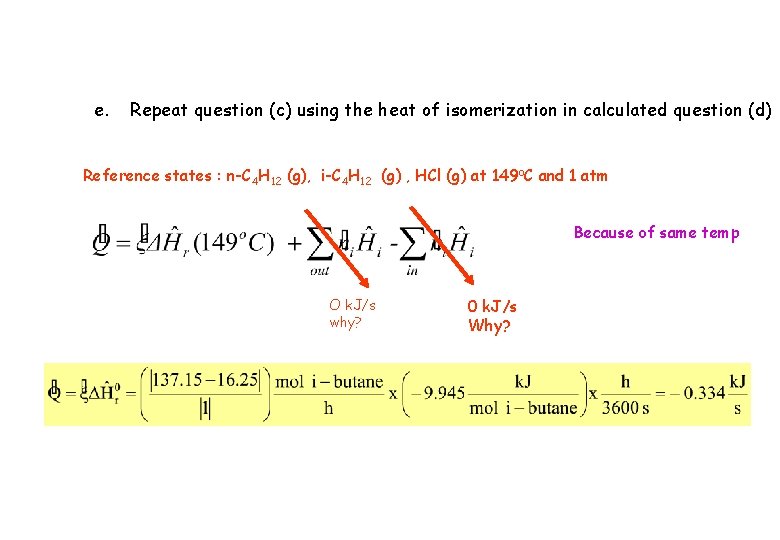
e. Repeat question (c) using the heat of isomerization in calculated question (d) Reference states : n-C 4 H 12 (g), i-C 4 H 12 (g) , HCl (g) at 149 o. C and 1 atm Because of same temp O k. J/s why? 0 k. J/s Why?
Example 9. 5. 2 Energy balance calculation - Heat of formation method - Heat of reaction method
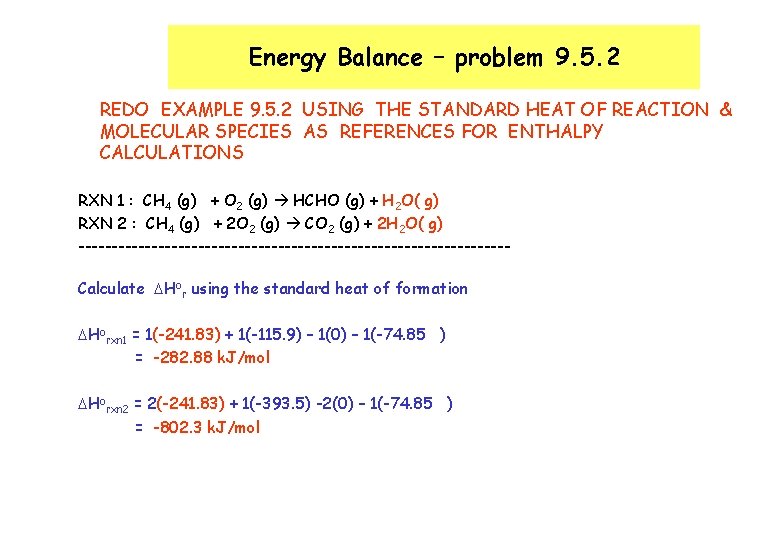
Energy Balance – problem 9. 5. 2 REDO EXAMPLE 9. 5. 2 USING THE STANDARD HEAT OF REACTION & MOLECULAR SPECIES AS REFERENCES FOR ENTHALPY CALCULATIONS RXN 1 : CH 4 (g) + O 2 (g) HCHO (g) + H 2 O( g) RXN 2 : CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O( g) --------------------------------Calculate DHor using the standard heat of formation DHorxn 1 = 1(-241. 83) + 1(-115. 9) – 1(0) – 1(-74. 85 ) = -282. 88 k. J/mol DHorxn 2 = 2(-241. 83) + 1(-393. 5) -2(0) – 1(-74. 85 ) = -802. 3 k. J/mol
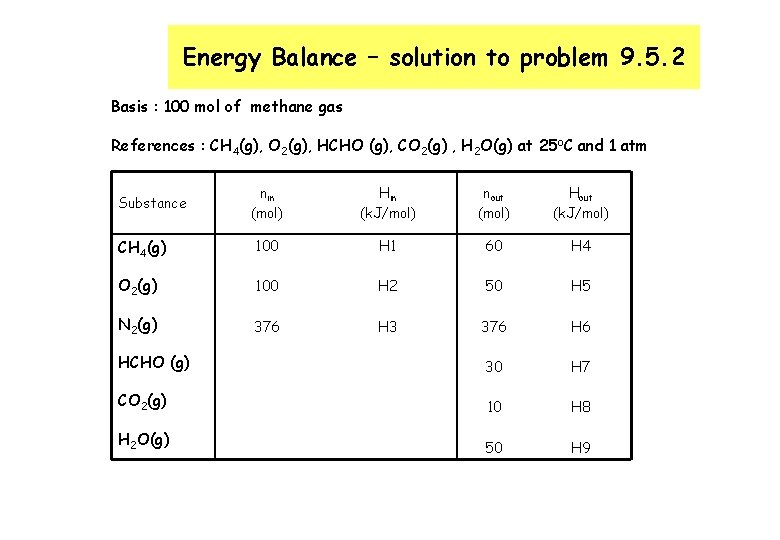
Energy Balance – solution to problem 9. 5. 2 Basis : 100 mol of methane gas References : CH 4(g), O 2(g), HCHO (g), CO 2(g) , H 2 O(g) at 25 o. C and 1 atm nin (mol) Hin (k. J/mol) nout (mol) Hout (k. J/mol) CH 4(g) 100 H 1 60 H 4 O 2(g) 100 H 2 50 H 5 N 2(g) 376 H 3 376 H 6 HCHO (g) 30 H 7 CO 2(g) 10 H 8 H 2 O(g) 50 H 9 Substance
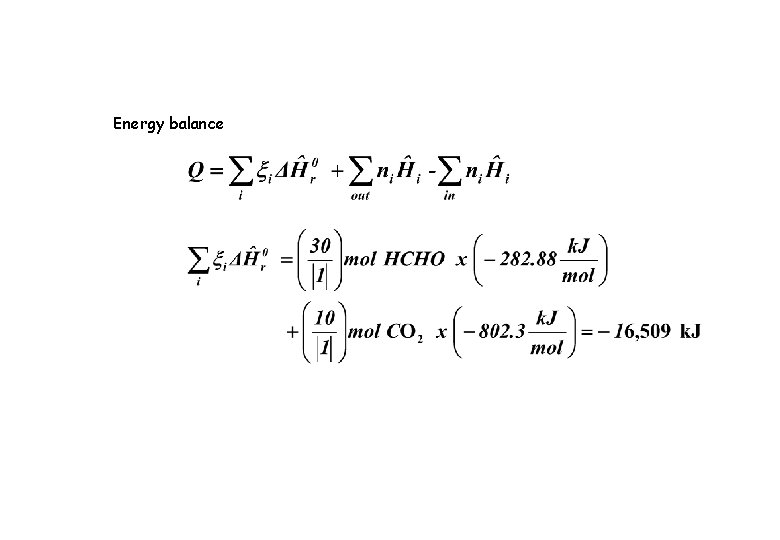
Energy balance

Entalphies are estimated using Tables B. 2 & B. 8
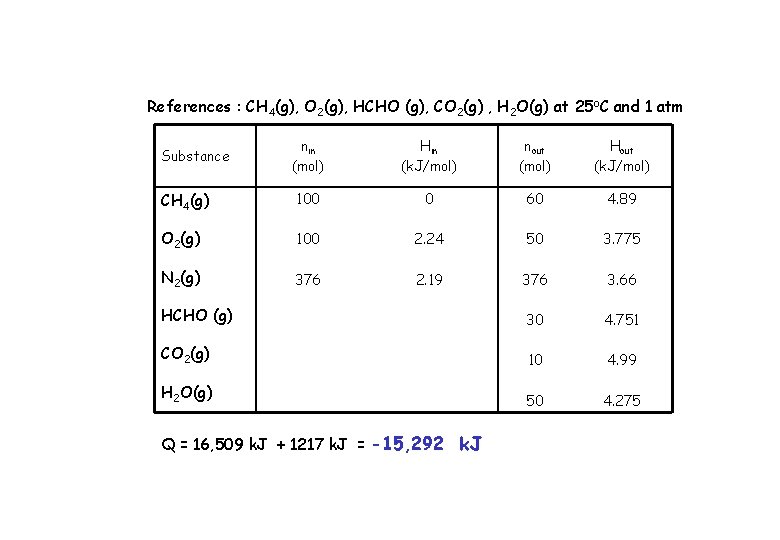
References : CH 4(g), O 2(g), HCHO (g), CO 2(g) , H 2 O(g) at 25 o. C and 1 atm nin (mol) Hin (k. J/mol) nout (mol) Hout (k. J/mol) CH 4(g) 100 0 60 4. 89 O 2(g) 100 2. 24 50 3. 775 N 2(g) 376 2. 19 376 3. 66 HCHO (g) 30 4. 751 CO 2(g) 10 4. 99 H 2 O(g) 50 4. 275 Substance Q = 16, 509 k. J + 1217 k. J = -15, 292 k. J
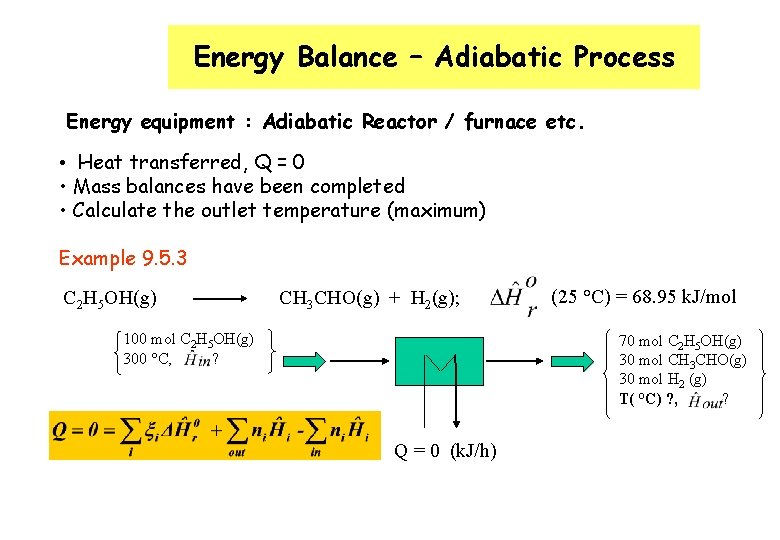
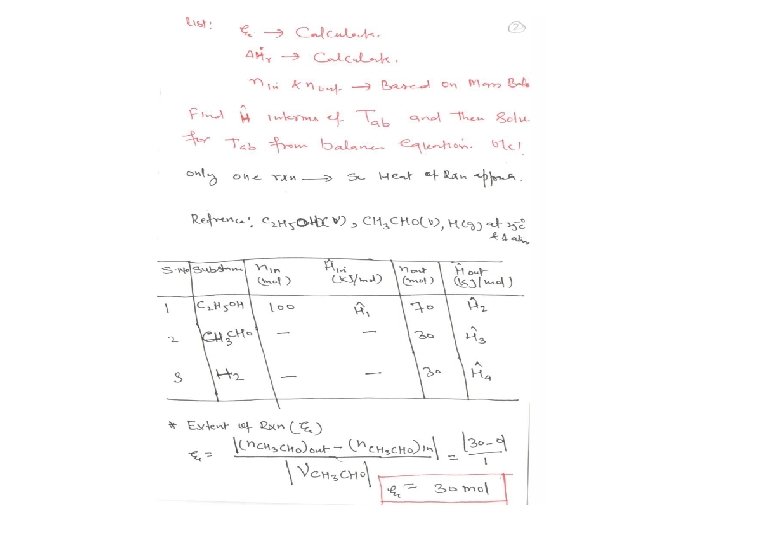
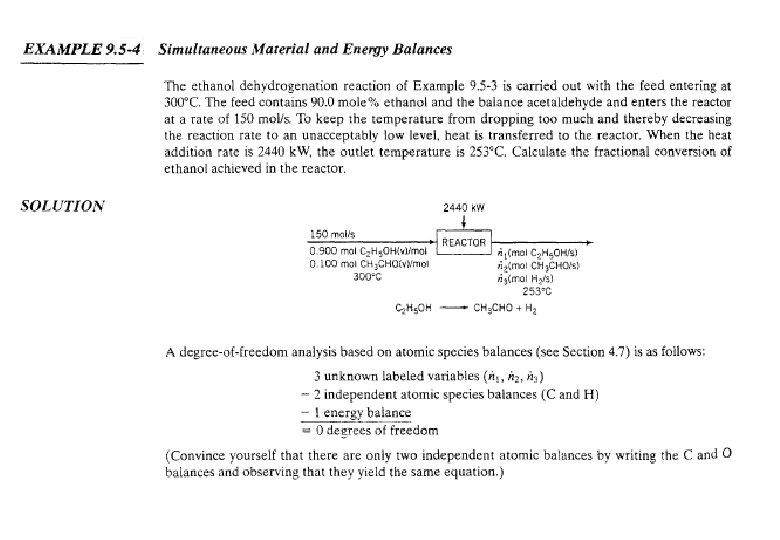
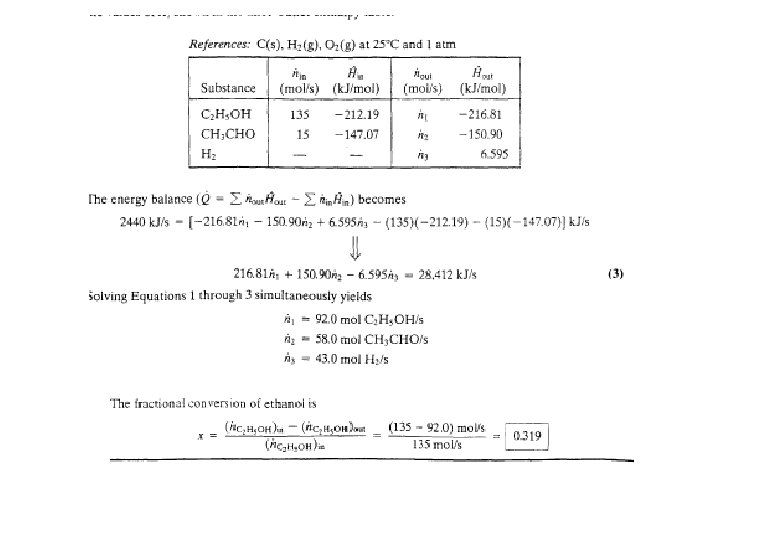
Energy Balance – Adiabatic Process Energy equipment : Adiabatic Reactor / furnace etc. • Heat transferred, Q = 0 • Mass balances have been completed • Calculate the outlet temperature (maximum) Example 9. 5. 3 C 2 H 5 OH(g) CH 3 CHO(g) + H 2(g); 100 mol C 2 H 5 OH(g) 300 °C, ? (25 °C) = 68. 95 k. J/mol 70 mol C 2 H 5 OH(g) 30 mol CH 3 CHO(g) 30 mol H 2 (g) T( °C) ? , ? Q = 0 (k. J/h)
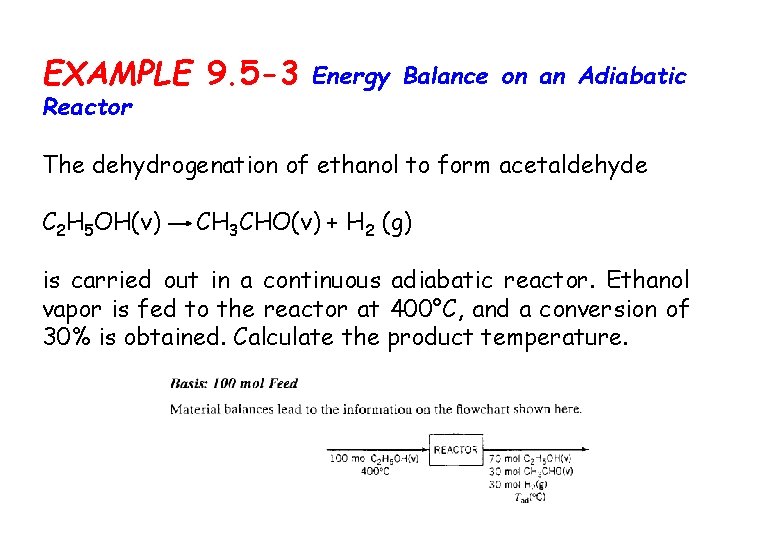
EXAMPLE 9. 5 -3 Reactor Energy Balance on an Adiabatic The dehydrogenation of ethanol to form acetaldehyde C 2 H 5 OH(v) CH 3 CHO(v) + H 2 (g) is carried out in a continuous adiabatic reactor. Ethanol vapor is fed to the reactor at 400°C, and a conversion of 30% is obtained. Calculate the product temperature.




- Slides: 51