Balance chemical reactions Start by choosing a species
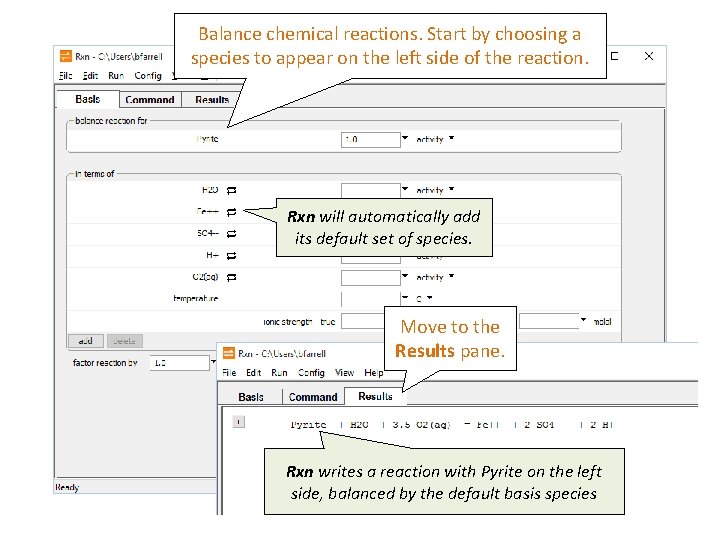
Balance chemical reactions. Start by choosing a species to appear on the left side of the reaction. Rxn will automatically add its default set of species. Move to the Results pane. Rxn writes a reaction with Pyrite on the left side, balanced by the default basis species
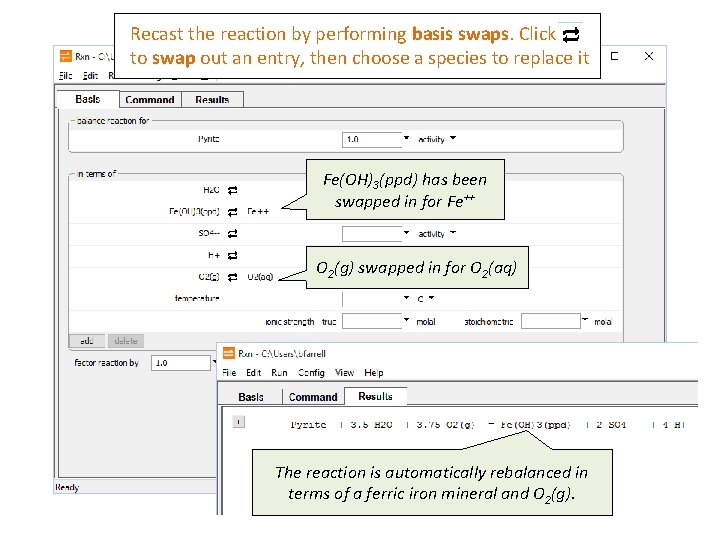
Recast the reaction by performing basis swaps. Click to swap out an entry, then choose a species to replace it Fe(OH)3(ppd) has been swapped in for Fe++ O 2(g) swapped in for O 2(aq) The reaction is automatically rebalanced in terms of a ferric iron mineral and O 2(g).
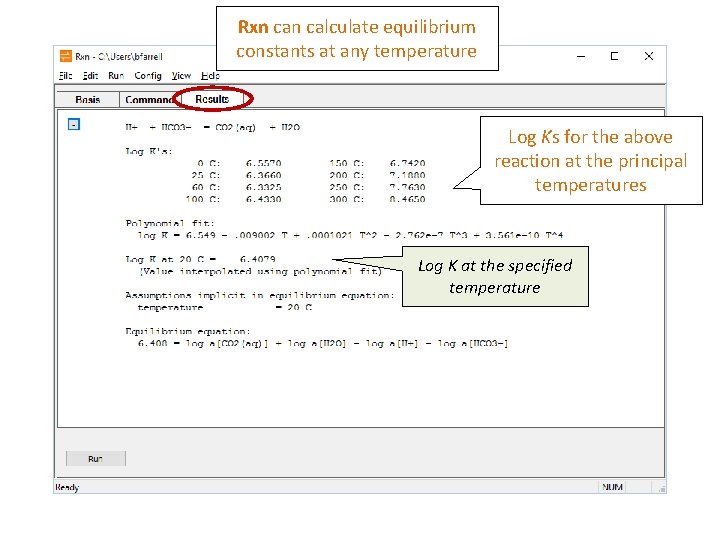
Rxn calculate equilibrium constants at any temperature Log Ks for the above reaction at the principal temperatures Log K at the specified temperature
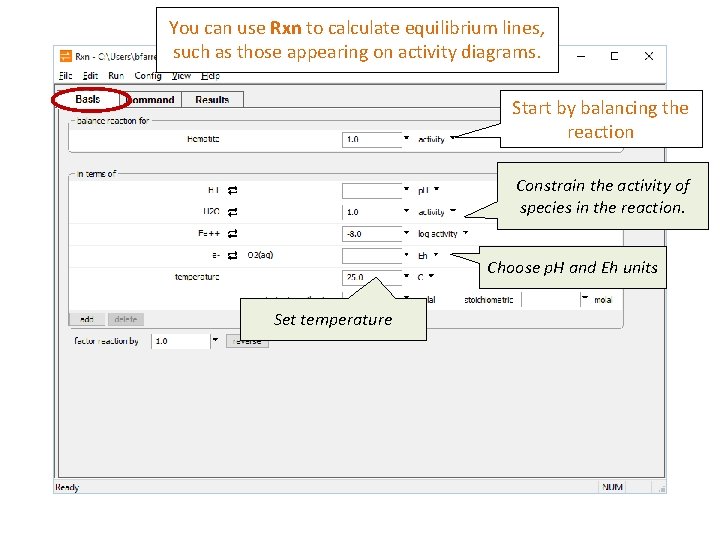
You can use Rxn to calculate equilibrium lines, such as those appearing on activity diagrams. Start by balancing the reaction Constrain the activity of species in the reaction. Choose p. H and Eh units Set temperature
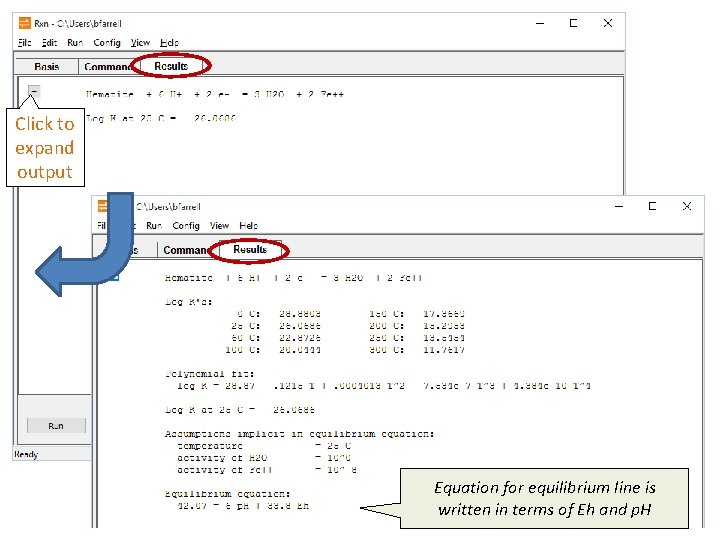
Click to expand output Equation for equilibrium line is written in terms of Eh and p. H
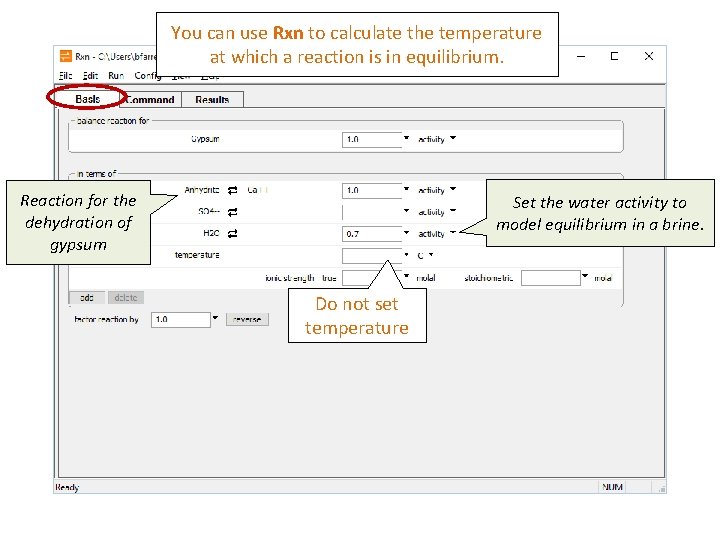
You can use Rxn to calculate the temperature at which a reaction is in equilibrium. Reaction for the dehydration of gypsum Set the water activity to model equilibrium in a brine. Do not set temperature
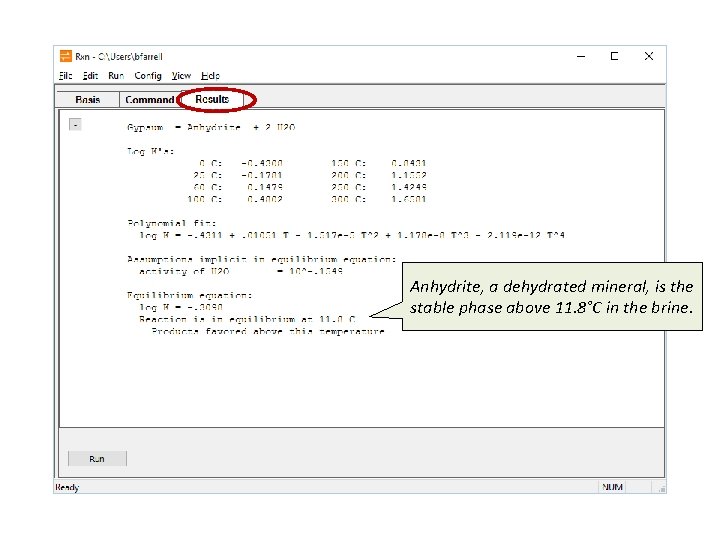
Anhydrite, a dehydrated mineral, is the stable phase above 11. 8°C in the brine.
- Slides: 7