Bacterial Metabolism Introduction Enzymes Energy Production Bacterial Catabolism

Bacterial Metabolism Introduction Enzymes Energy Production Bacterial Catabolism
Bacterial Metabolism l Introduction – Metabolism - sum of all chemical reactions in cell – Anabolism - reactions that synthesize or “build up” e. g. protein synthesis – Catabolism - reactions that digest or “break down” e. g. starch to glucose
Bacterial Metabolism Enzyme Introduction l Enzyme Components l Enzyme Mechanism l Factors Influencing Enzymes l
Bacterial Metabolism l Enzyme Introduction – Enzymes are biological catalysts – Catalysts are agents which speed up a reaction – Enzymes are very specific – Enzymes are typically proteins – Catalysts work by lowering the activation energy of a reaction
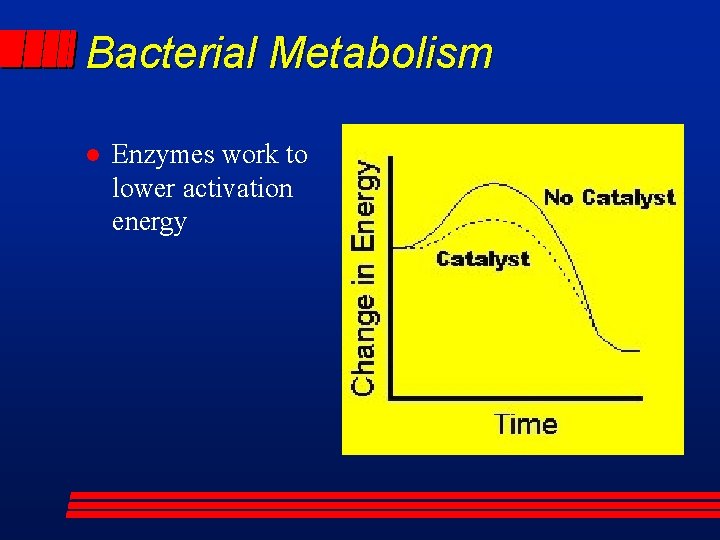
Bacterial Metabolism l Enzymes work to lower activation energy
Bacterial Metabolism l Enzyme Components – Cofactor - nonprotein component that is part of enzyme, e. g. Fe, NAD+, biotin – Apoenzyme - protein portion of enzyme – Holoenzyme - Cofactor plus apoenzyme
Bacterial Metabolism l How enzymes speed up reactions – Proximity – Orientation – Induced fit – Reactive groups – Cofactors
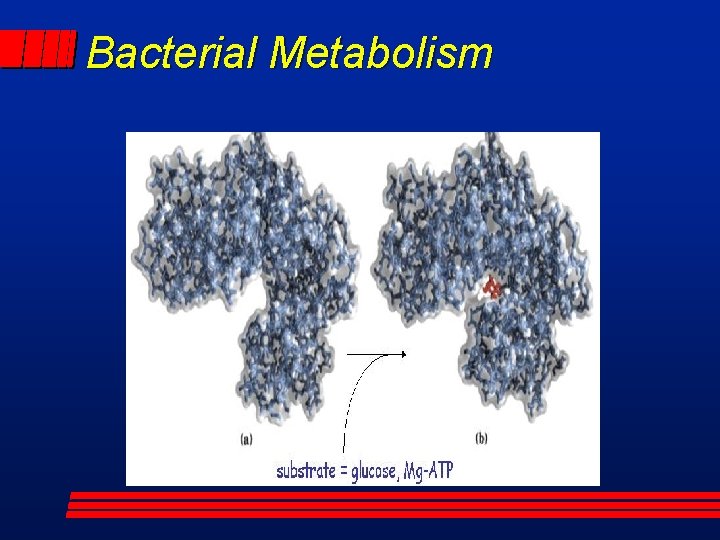
Bacterial Metabolism l Enzyme Mechanism – Substrate binds to active site; lock & key specificity; induced fit – Formation of enzyme-substrate complex – Catalytic activity; localized acid or base or induced fit
Bacterial Metabolism
Bacterial Metabolism l Factors Influencing Enzymes – Temperature – p. H – Salt concentration – Inhibitors » Competitive (active site) » Non - Competitive (allosteric) – Feedback Inhibition
Bacterial Metabolism l Energy Production – Oxidation / Reduction reactions – Role of ATP – Phosphorylation » Substrate » Oxidative » Photo-
Bacterial Metabolism l Oxidation / Reduction – Oxidation - loss of electrons – Reduction - gain of electrons – Redox reactions always coupled – Oxidation of reduced carbon tends to be energetically favorable
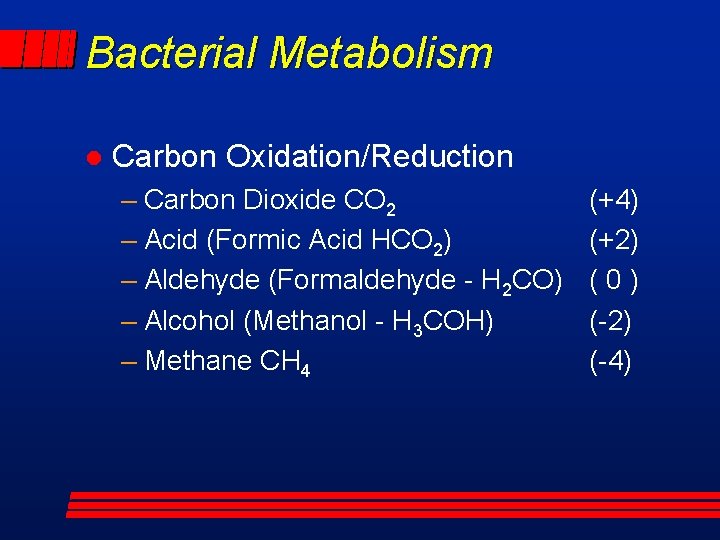
Bacterial Metabolism l Carbon Oxidation/Reduction – Carbon Dioxide CO 2 – Acid (Formic Acid HCO 2) – Aldehyde (Formaldehyde - H 2 CO) – Alcohol (Methanol - H 3 COH) – Methane CH 4 (+4) (+2) (0) (-2) (-4)
Bacterial Metabolism l Oxidation States – Alcohols – Fats – Organic Acids (acetic acid) – Glucose
Bacterial Metabolism l Role of ATP – ATP ADP + Pi – Energy intermediate or “currency” – Hydrolysis of ATP “coupled” to energetically unfavorable reactions
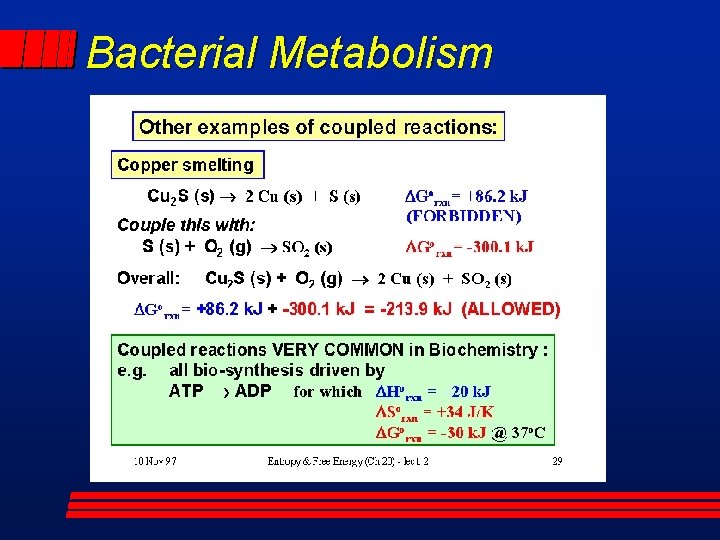
Bacterial Metabolism
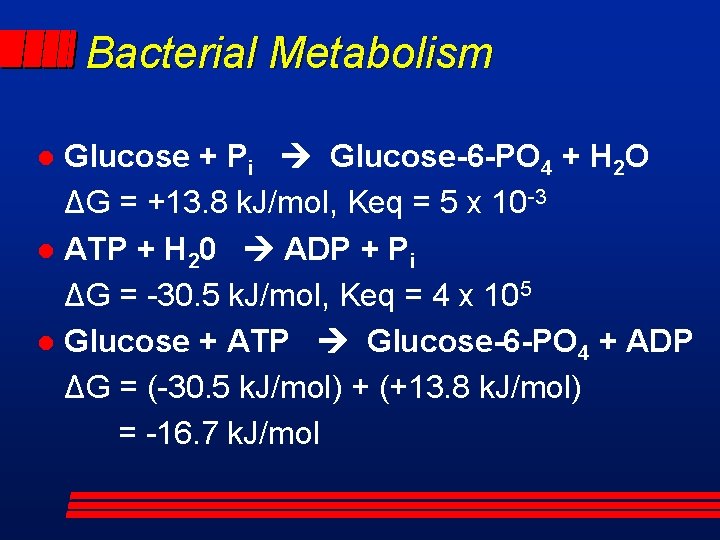
Bacterial Metabolism Glucose + Pi Glucose-6 -PO 4 + H 2 O ΔG = +13. 8 k. J/mol, Keq = 5 x 10 -3 l ATP + H 20 ADP + Pi ΔG = -30. 5 k. J/mol, Keq = 4 x 105 l Glucose + ATP Glucose-6 -PO 4 + ADP ΔG = (-30. 5 k. J/mol) + (+13. 8 k. J/mol) = -16. 7 k. J/mol l
Bacterial Metabolism l Phosphorylation – Substrate - direct transfer of phosphate from an organic molecule to ADP – Oxidative - ATP generated via chemiosmosis (“proton pump”) and ATP synthase – Photo - light energy from photosynthesis, a modification of chemiosmosis
Bacterial Metabolism l Bacterial Catabolism – Carbohydrate catabolism has two functions: » energy production and/or storage » generation of chemical intermediates – Cellular respiration and fermentation – Includes three processes: » Glycolysis » Kreb’s or Tricarboxylic Acid (TCA) cycle » Electron transport /oxidative phosphorylation
- Slides: 19