Background Human immunodeficiency virus HIV1 33 million people

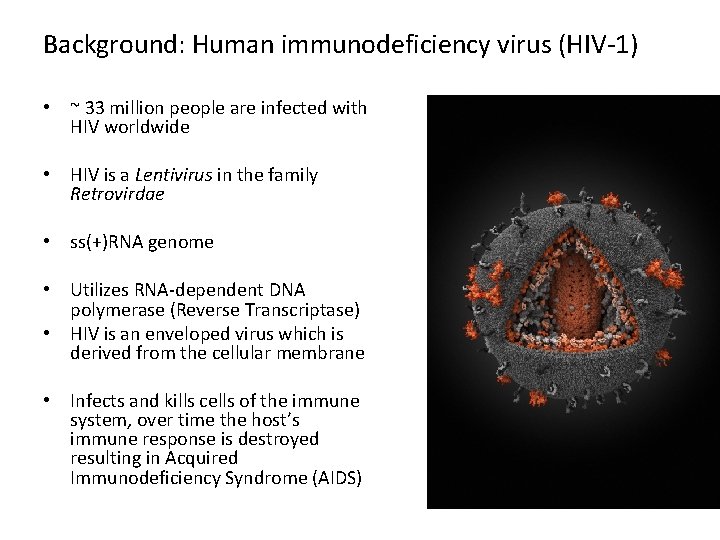
Background: Human immunodeficiency virus (HIV-1) • ~ 33 million people are infected with HIV worldwide • HIV is a Lentivirus in the family Retrovirdae • ss(+)RNA genome • Utilizes RNA-dependent DNA polymerase (Reverse Transcriptase) • HIV is an enveloped virus which is derived from the cellular membrane • Infects and kills cells of the immune system, over time the host’s immune response is destroyed resulting in Acquired Immunodeficiency Syndrome (AIDS)
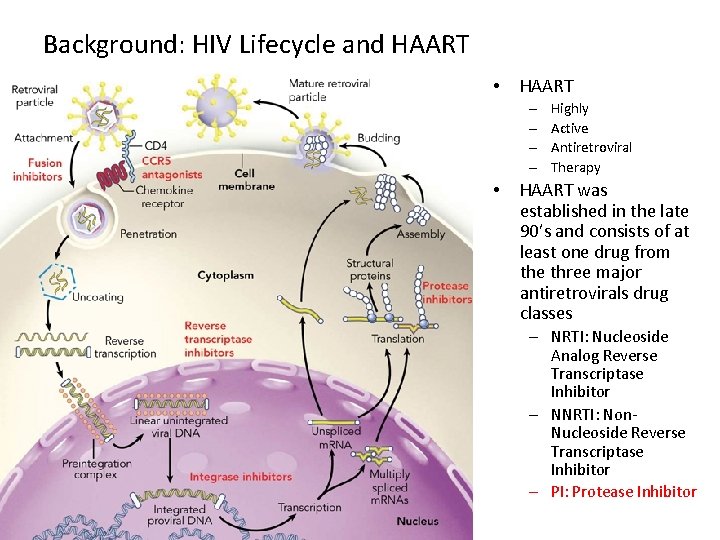
Background: HIV Lifecycle and HAART • HAART – – • Highly Active Antiretroviral Therapy HAART was established in the late 90’s and consists of at least one drug from the three major antiretrovirals drug classes – NRTI: Nucleoside Analog Reverse Transcriptase Inhibitor – NNRTI: Non. Nucleoside Reverse Transcriptase Inhibitor – PI: Protease Inhibitor
Background: HIV and Cholesterol • HIV and cholesterol: – HIV enters the host cell at lipid rafts – Treatment of cells with lovastatin (inhibitor of cholesterol synthesis, HMGCo. A) inhibits HIV entry – Treatment of cells with oxysterol (inhibitor of cholesterol synthesis, SREBP 2) inhibits HIV replication – HIV protein Nef, binds and transports cholesterol to the cellular membrane during viral particle assembly, also increases cholesterol influx – HIV infection ALONE, is associated with hypocholesterolemia and hypertriglyceridemia – HIV infected individuals show a 3 - to 4 - fold increase in de novo hepatic lipogenesis compared to HIV negative controls
Background: HAART-associated Dyslipidemia • Long-term exposure to HAART can cause severe metabolic side effects – Lypodystrophy (body fat distribution) – Dyslipidemia (alterations in serum lipid levels) • Dyslipidemia in people receiving HAART varies by degree and prevalence, but is most severe in those individuals who also have lypodystrophy – Increased serum lipid levels compared to pre-seroconversion • • Total Cholesterol (TCHOL) Low-Density Lipoprotein Cholesterol (LDL) Triglycerides (TRIG) Very-Low-Density Lipoprotein Cholesterol (VLDL) – Decreased compared to pre-seroconversion levels • High-Density Lipoprotein Cholesterol (HDL)
Research Question and Hypothesis “ Do genetic variations in human genes involved in lipid metabolism contribute to the development of HAARTassociated dyslipidemia in HIV-positive individuals? ” “If genetic variations in lipid metabolism genes contribute to HAART-associated dyslipidemia, then we would expect dyslipidemia to vary by biogeographical ancestry in HIV positive individuals on HAART. ”
Materials and Methods • Study population – Peripheral Blood Mononuclear Cell (PBMC) pellets were obtained from 1, 945 men participating in the Multicenter AIDS Cohort Study (MACS) – Patient visit data, including lipid values, were obtained for these individuals spanning over thirties years • Genotyping – DNA was extracted using Qiagen QIAmp DNA Blood mini kit – 1, 536 SNPs were chosen from known SNPs that are associated with serum cholesterol levels and a subset of SNPs used to define biogeographical ancestry – Ancestry Informative Markers (AIMs) – Genotyping was performed at the University of Pittsburgh Genomics and Proteomics Core Laboratory using a Illumina Golden. Gate array

Materials and Methods • Biogeographical Ancestry (BGA) – Using the panel of AIMs, multidimensional scaling (MDS) was performed to cluster individuals based on the correlation of the AIMs using PLINK – Reference populations were downloaded from Hap. Map to identify clustering of known BGA groups – Three BGA groups were identified using k-means clustering algorithm, the individuals of European ancestry and African/European admixed ancestry were chosen to use for the study (n= 1, 779)
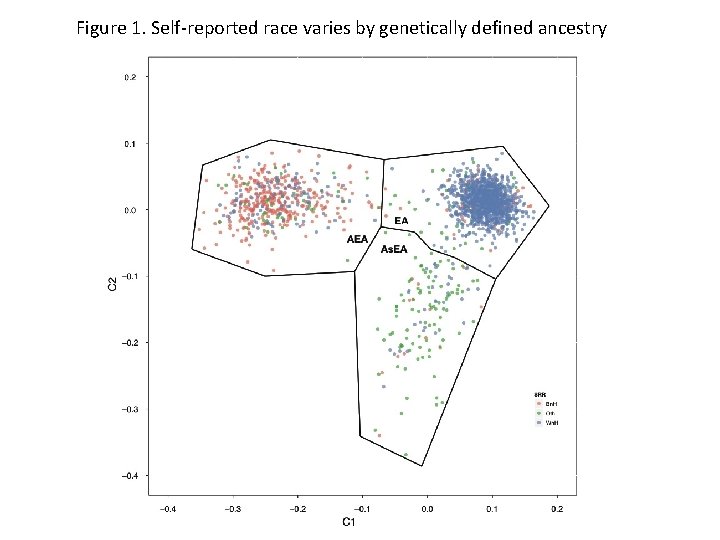
Figure 1. Self-reported race varies by genetically defined ancestry
Materials and Methods • Lipid Measurements and Covariate Data – Longitudinal patient visits (PV) on relevant covariate data was obtained: • Age, BMI, SRR, HIV-1 serostatus, type of HAART, HAART duration, HAART adherence, HIV-1 viral load, etc… – PV data for four lipid phenotypes (LDL-C, HDL-C, Trig, Total Cholesterol) were taken from visits patients reported fasting for >8 hrs and not taking statins.
Materials and Methods • Statistical Analysis – To account for the repeated measures of the PV data a Mixed-effects linear regression analysis was used – Hypothesis testing was performed using a Wald Test and parameters were estimated using maximum likelihood – The effects of covariates on lipid phenotypes was assessed using univariate and multivariate models – Continuous variables (age, BMI, etc. ) were centered to make model intercepts meaningful – Triglyceride values were log transformed before analysis – STATA 12 and R software packages were used
Results: Study Demographics • BGA and SRR showed a 85% concordance for European Ancestry and White non-Hispanics • BGA and SRR showed a 69% concordance for African/European Ancestry and Black non. Hispanics
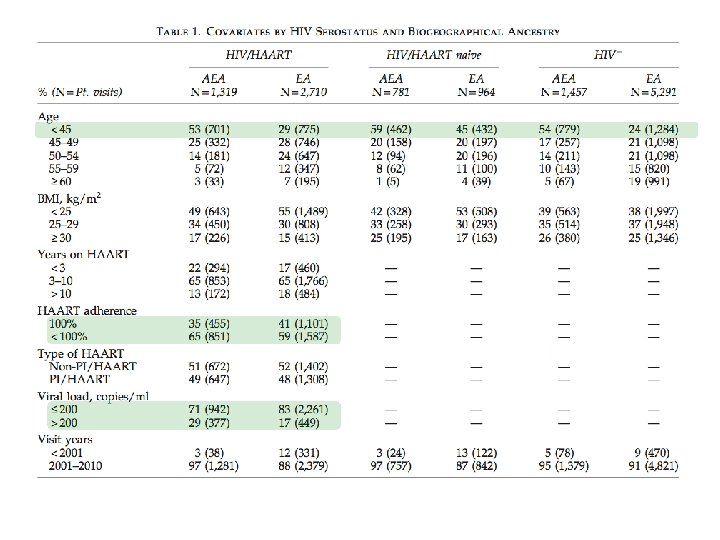

Figure 2. Mean serum lipid values vary by BGA and HIV/HAART Status Blue = Raw Means Red = Adjusted Means
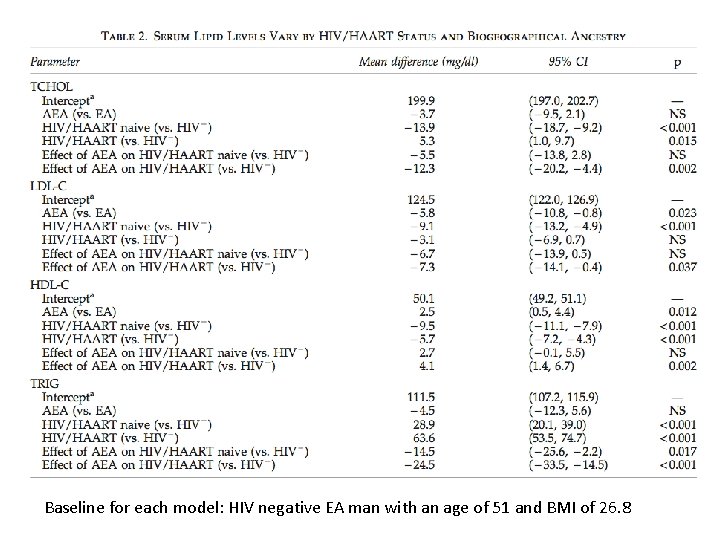
Baseline for each model: HIV negative EA man with an age of 51 and BMI of 26. 8
Results: Conclusions Figure/Table 2 • Lipid levels vary by BGA and HIV/HAART Status – On average, HIV+/HAART+ individuals had significantly different mean lipid levels compared to HIV- individuals… except for LDL-C – BGA interacts with HIV/HAART status for all lipid phenotypes… that is, the differences identified in the above conclusion are different by BGA
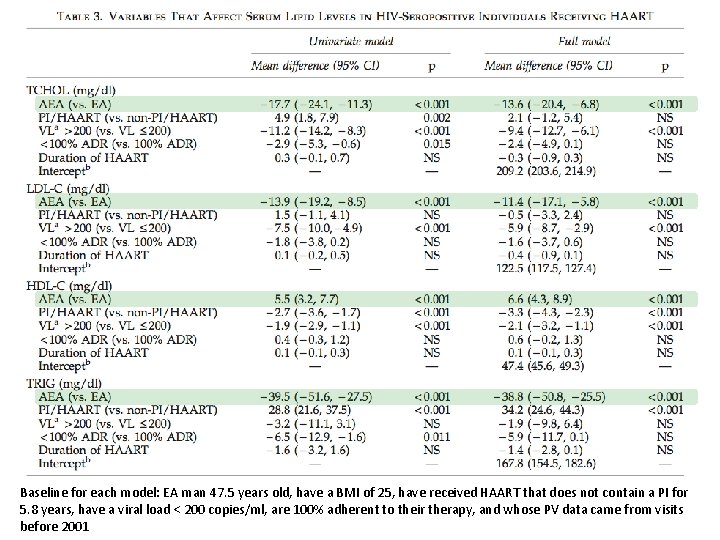
Baseline for each model: EA man 47. 5 years old, have a BMI of 25, have received HAART that does not contain a PI for 5. 8 years, have a viral load < 200 copies/ml, are 100% adherent to their therapy, and whose PV data came from visits before 2001
Discussion/Conclusions • HIV/HAART individuals have significantly different lipid profiles than HIV- controls • The atherogenic effects of chronic HAART exposure differs by BGA and is more severe in EA men than AEA men – Higher mean triglycerides – Lower mean HDL-C
- Slides: 18