Background Chemical reactions involves the making and breaking
Background • Chemical reactions involves the making and breaking of bonds. • To better understand the energy changes in a reaction, will look at the energy and enthalpy changes in a reaction as bonds are broken in the reactants and formed in the products • Remember what a bond is? = energy needed to break the bonds in one mole of a gaseous molecule under standard conditions
5. 3 Bond enthalpies Understandings: • Bond formed= releases energy • Bond broken= requires energy is the energy needed to break one mole of a bond in a gaseous molecule averaged over similar compounds Section 11 of data booklet
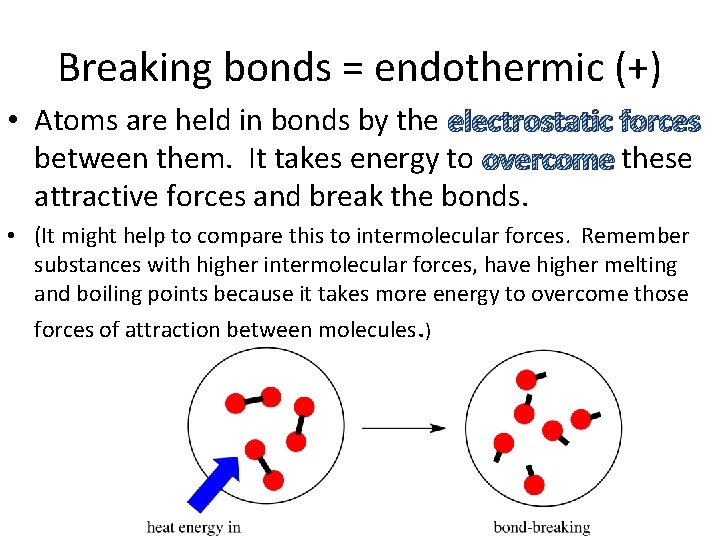
Breaking bonds = endothermic (+) • Atoms are held in bonds by the electrostatic forces between them. It takes energy to overcome these attractive forces and break the bonds. • (It might help to compare this to intermolecular forces. Remember substances with higher intermolecular forces, have higher melting and boiling points because it takes more energy to overcome those forces of attraction between molecules. )
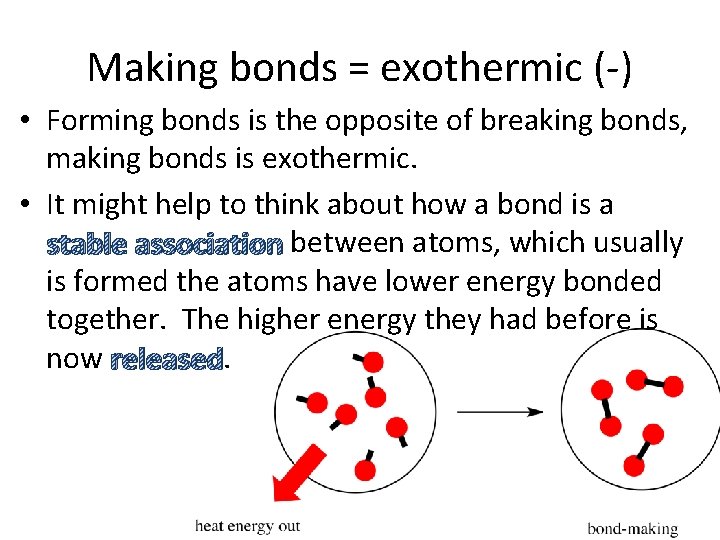
Making bonds = exothermic (-) • Forming bonds is the opposite of breaking bonds, making bonds is exothermic. • It might help to think about how a bond is a stable association between atoms, which usually is formed the atoms have lower energy bonded together. The higher energy they had before is now released.
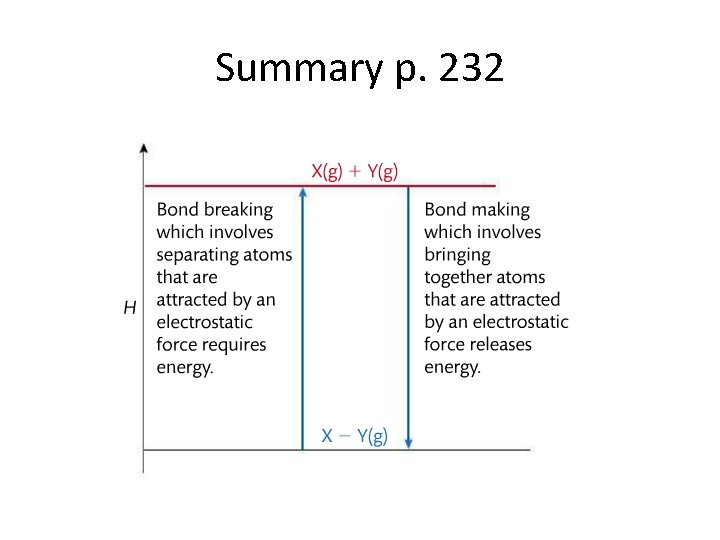
Summary p. 232
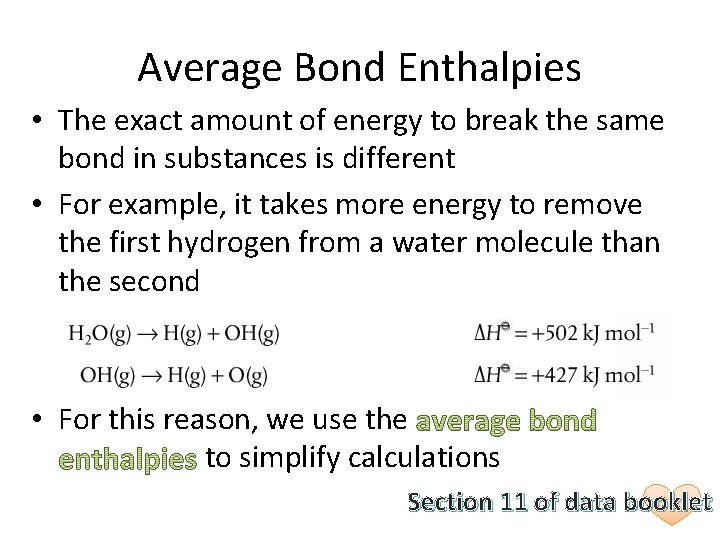
Average Bond Enthalpies • The exact amount of energy to break the same bond in substances is different • For example, it takes more energy to remove the first hydrogen from a water molecule than the second • For this reason, we use the to simplify calculations Section 11 of data booklet
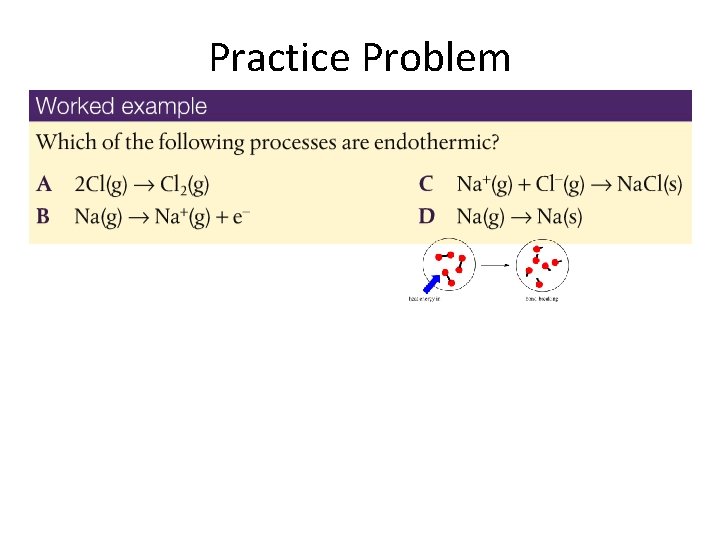
Practice Problem
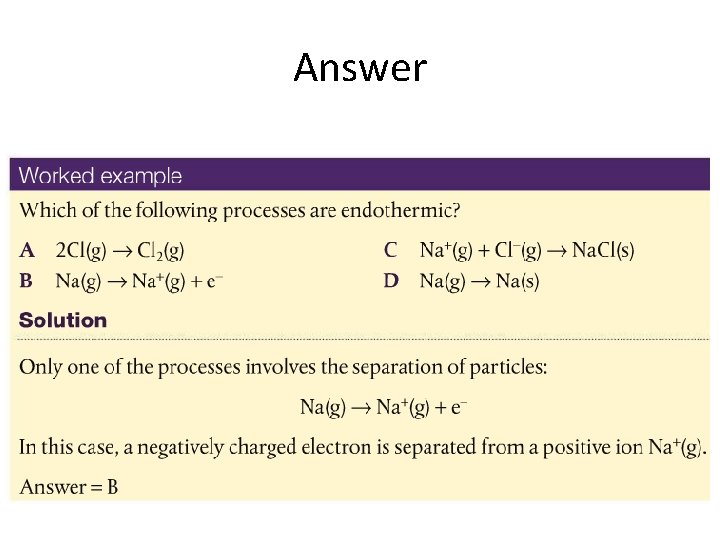
Answer
Using bond enthalpies to calculate the enthalpy changes of reactions • We can now hopefully understand which processes are endothermic and exothermic. • We must remember to look at the bonds formed and broken. • Write the balanced chemical equation for the combustion of methane gas, and then draw out the Lewis structures below, counting how many of each type of bond are broken and formed.
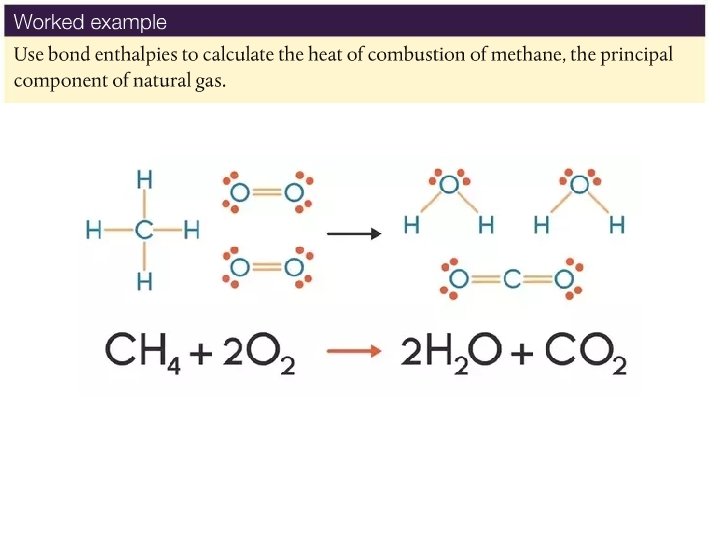
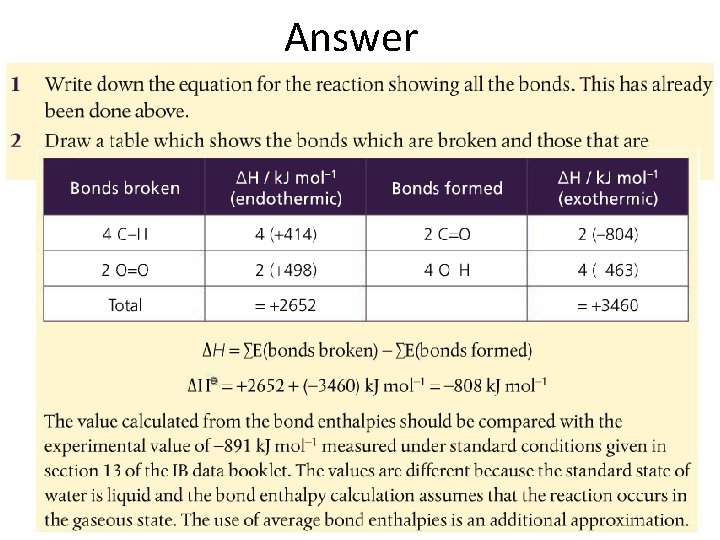
Answer
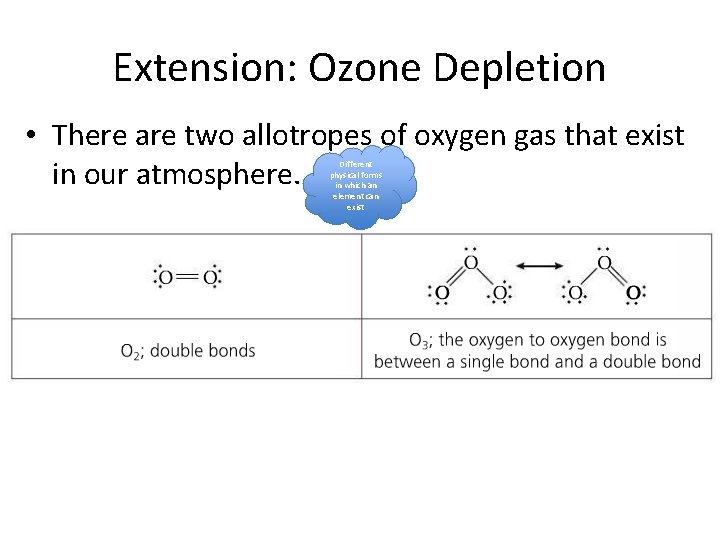
Extension: Ozone Depletion • There are two allotropes of oxygen gas that exist in our atmosphere. Different physical forms in which an element can exist
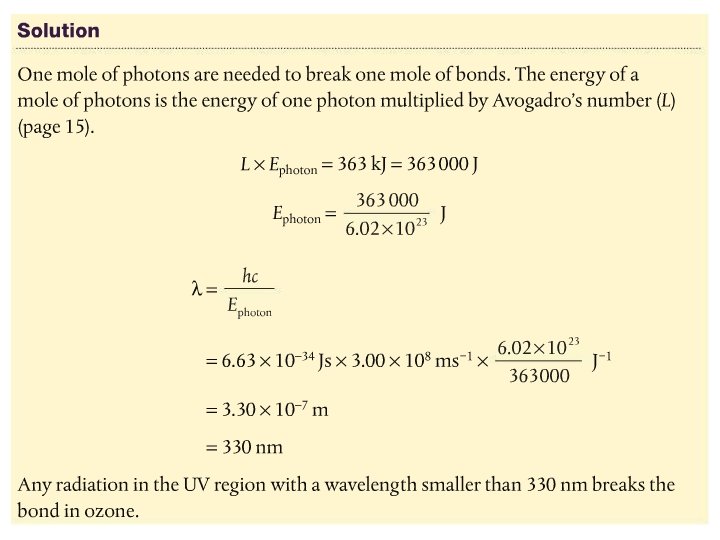
The bonds in oxygen and ozone are broken by UV of different wavelengths • It takes more energy to break the double bond in O 2 than the 1. 5 bonds in O 3 • Physics! Section 2 of data booklet

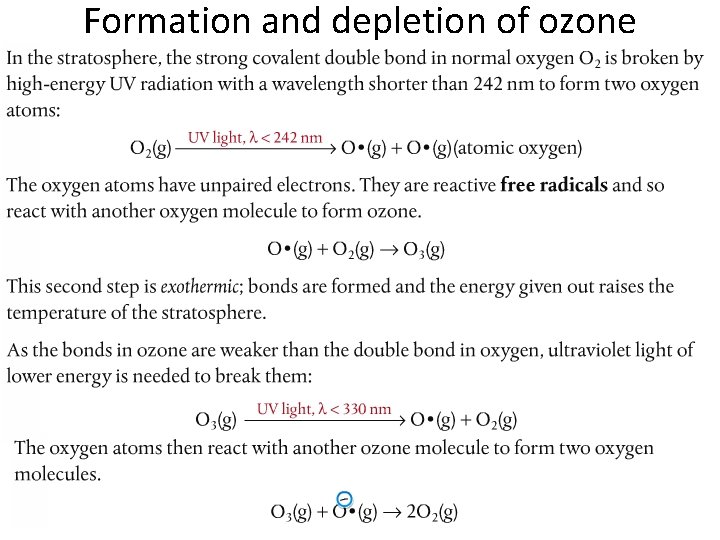
Formation and depletion of ozone
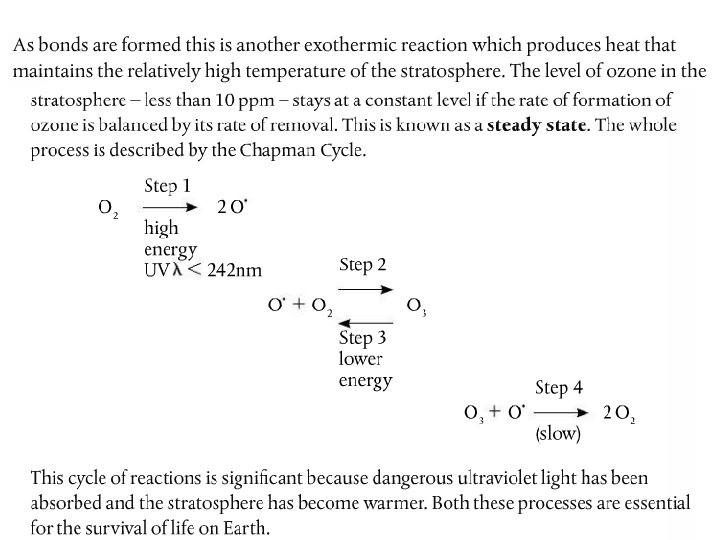

- Slides: 18