Background 12 5 million people around the globe
Background • 12. 5 million people around the globe affected by Polycystic Kidney Disease • Cyst number and growth correlates with disease progression • VP 2 R Antagonist Tolvaptan first drug to slow CKD progression via reducing cyst growth, but limited efficacy and relevant side effects • Pharmacotherapeutic approaches to date: Candidate drug selection based on mode of action • Alternative approach: High-Throughput Phenotype Screening of Compound Libraries
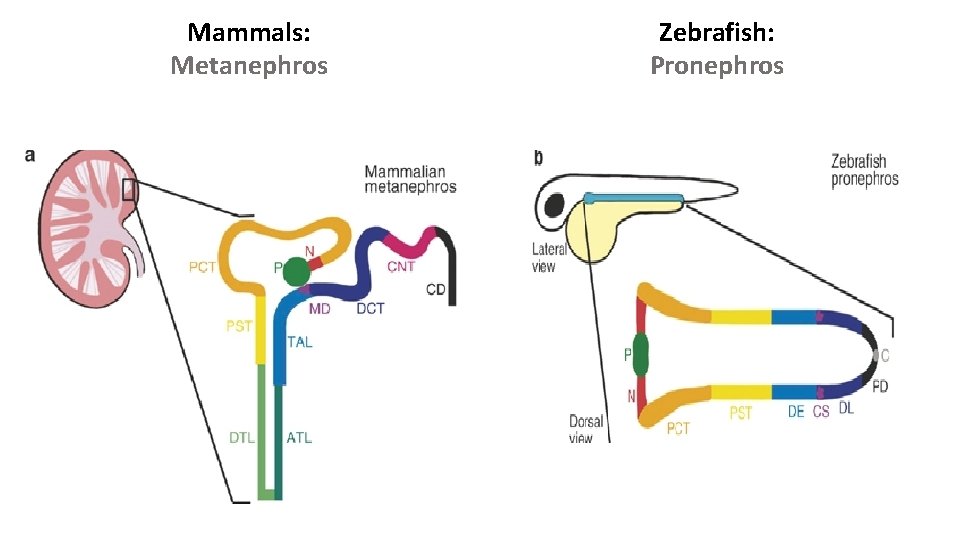
Mammals: Metanephros Zebrafish: Pronephros
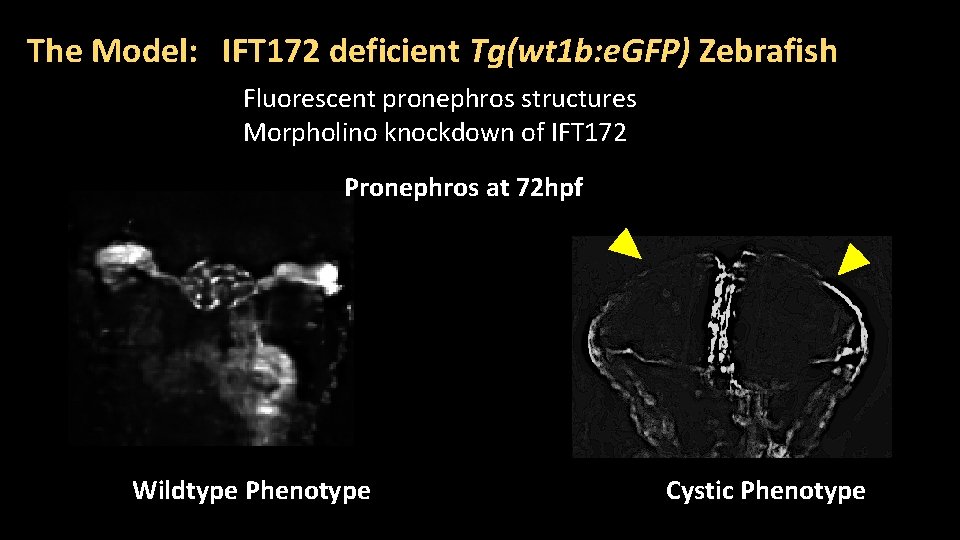
The Model: IFT 172 deficient Tg(wt 1 b: e. GFP) Zebrafish Fluorescent pronephros structures Morpholino knockdown of IFT 172 Pronephros at 72 hpf Wildtype Phenotype Cystic Phenotype
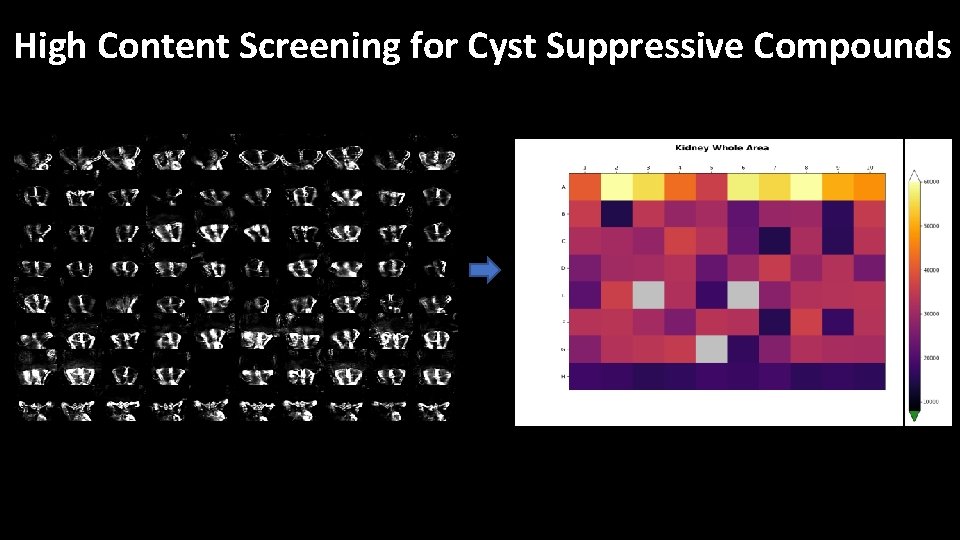
High Content Screening for Cyst Suppressive Compounds
Prestwick Compound Library Collection of 1280 molecules comprising 100% FDA/EMA-approved drugs selected for their high chemical and pharmacological diversity. These off-patent drugs have known bioavailability and safety data in humans are available.
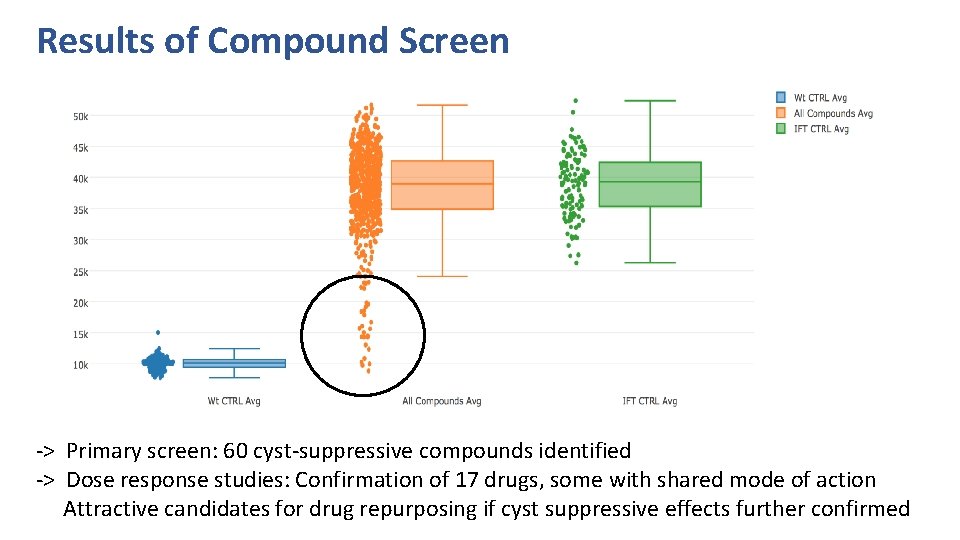
Results of Compound Screen -> Primary screen: 60 cyst-suppressive compounds identified -> Dose response studies: Confirmation of 17 drugs, some with shared mode of action Attractive candidates for drug repurposing if cyst suppressive effects further confirmed

Required Next Steps • Test candidate compounds in murine/human 3 D culture models • Test candidate compounds in rodent PKD models -> Need for partner with expertise in mammalian in vitro cystic kidney models
Interaction with EATRIS • Initial contact with EATRIS • Letter of Engagement signed • Research proposal submitted • Matchmaking report received within 2 weeks: 1 excellent match Dr. Christodoulos Xinaris Laboratory of Organ Regeneration Istituto di Ricerche Farmacologiche Mario Negri, Milan, Italy
“Using PDMS scaffolds and kidney cells, we recently developed a system for rapidly engineering complex 3 D kidney tubules with the typical structural and functional features of renal epithelium. We have then successfully used this system to generate patient-derived polycystic tubules to model Polycystic Kidney Disease (PKD) and test drug efficacy quantitatively. We can offer our expertise and technology to test the efficacy of selected compounds on human polycystic tubules and confirm zebrafish findings. This will provide a proof-of-principle treatment for human PKD. To this end, • we will engineer patient-derived polycystic tubules and culture them in the presence of pharmacologically active compounds (alone or in combination). • We will then evaluate the drugs’ anticystogenic capacities by quantifying cyst number/mm 2 and cystic area in treated tubules compared to untreated controls. • To evaluate drug efficacy at the morphological and molecular level, we will analyse epithelial organisation and polarisation and the integrity of the lumen, using histology and immunofluorescence approaches. • Finally, we will evaluate changes in the signalling pathways that are involved in cell
- Slides: 9