Back to the Solution Basics Stoichiometry Qualitative Analysis
Back to the Solution Basics Stoichiometry Qualitative Analysis/ Ionic Equation Solubility Curves Acids and Bases/ Titration $100 $100 $200 $200 $300 $300 $400 $400 $500 $500

How many grams of bromine, Br 2, are needed to prepare 0. 500 L of a 0. 0100 M solution in water?

Answer: 0. 8 grams 1. Find the number of moles of Bromine gas: n=c*v n=0. 500 L*0. 0100 M n=0. 005 moles of Br 2 2. Covert moles into grams using the following triangle: 3. m=n*M Note: m=0. 005*160 MBr =2(80) m=0. 8 grams =160 g/mol 2 m n M

1. 2. 3. What is the difference between saturated, unsaturated and supersaturated solution? What is a solute and solvent? Distinguish the difference between concentration and dilution.

Solution: 1. Saturated solution: A solution that contains the maximum amount of solute that can be dissolved within a given volume. Unsaturated solution: A solution that doesn’t contain the maximum amount of solute, thus requiring more, in order to reach its saturation point. Supersaturated solution: A solution containing more than the maximum amount of solute that can be sustained in a given volume. 2. Solute: The substance that one adds into a solution Solvent: A liquid substance that dissolves the solute 3. Concentration: A solution that contains a lot of solute Dillution: Reducing the amount of solute in a solution that is concentrated.

Classify the following as Polar or Non Polar and identify the type of bond holding it together. 1. 2. 3. 4. 5. 6. 7. 8. C 2 H 2 C 2 H 4 CH 4 NH 3 H 2 O HBr HF CCl 3

Solution: 1. 2. 3. 4. 5. 6. 7. 8. C 2 H 2 Non Polar, London Dispersion C 2 H 4 Non Polar, London Dispersion CH 4 Non Polar, London Dispersion NH 3 Polar, Dipole H 2 O Polar, Hydrogen Bonding HBr Polar, Dipole HF Polar, Hydrogen Bonding CCl 3 Polar, Dipole

A saturated solution of calcium sulfate contains 0. 209 grams of solute in 100 m. L of solution. Calculate the molar concentration of the calcium sulfate solution.

Answer: 0. 015 M 1000 m. L=1 L Calcium sulfate Ca. SO 4 1. What is given? c=? , n=? , m=0. 209 g , v=0. 1 L 2. Find moles. Note: n= m/M MCa. SO =40+32+4(16) n=0. 209 g/ MCa. SO =136 g/mol n=0. 209/136 Note: n=0. 0015 mol Make sure to 3. Find concentration. ALWAYS convert c=n/v milliliters into liters for c=0. 0015 mol/0. 1 L volume because molarity is always in c=0. 015 mol/L 4 4 units of moles per liter (mol/L)

How many milliliters of 5. 5 M Na. OH are needed to prepare 300 m. L of 1. 2 M Na. OH?

Answer: 65 m. L Solution: 1. Use the dillution formula C 1 V 1= C 2 V 2 2. What is given? C 1= 5. 5 M, V 1= ? , C 2= 1. 2 M, V 2 = 300 m. L 3. Substitute each given value into the equation and solve for V 1 4. C 1 V 1= C 2 V 2 (5. 5 M)*V 1 = (1. 2 M)*(0. 3 L) Note: V = [(1. 2 M)*(0. 3 L)]/5. 5 M 1 Make sure to V = 0. 065 L 1 ALWAYS convert 5. Convert 0. 065 L into m. L milliliters into liters for 6. 1 L=1000 m. L volume because 0. 065 L=x m. L molarity is always in 7. Solve for x by cross multiplying. units of moles per liter 8. 1 L=1000 m. L (mol/L). 0. 065 L=x m. L x=1000 m. L*0. 065 L x=65 m. L

If you add 500 m. L of 0. 100 M Ag. NO 3 solution to a solution containing an excess of chlorine ion. How much moles of Ag. Cl precipitate will you form?
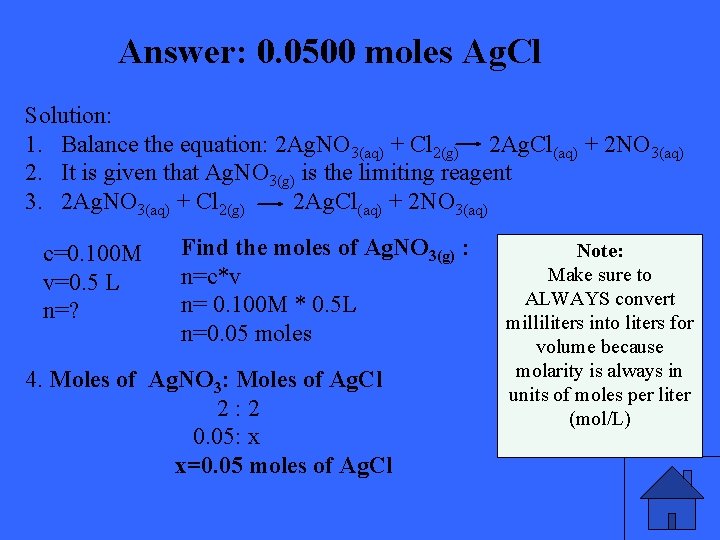
Answer: 0. 0500 moles Ag. Cl Solution: 1. Balance the equation: 2 Ag. NO 3(aq) + Cl 2(g) 2 Ag. Cl(aq) + 2 NO 3(aq) 2. It is given that Ag. NO 3(g) is the limiting reagent 3. 2 Ag. NO 3(aq) + Cl 2(g) 2 Ag. Cl(aq) + 2 NO 3(aq) c=0. 100 M v=0. 5 L n=? Find the moles of Ag. NO 3(g) : n=c*v n= 0. 100 M * 0. 5 L n=0. 05 moles 4. Moles of Ag. NO 3: Moles of Ag. Cl 2 : 2 0. 05: x x=0. 05 moles of Ag. Cl Note: Make sure to ALWAYS convert milliliters into liters for volume because molarity is always in units of moles per liter (mol/L)

If you mix 200 m. L of 0. 100 M Pb(NO 3)2 and 300 m. L of 0. 200 M Mg. Cl 2, how much moles of Pb. Cl 2 precipitate will you form?

Answer: 0. 0200 moles Pb. Cl 2 Solution: Pb(NO 3)2 + Mg. Cl 2 Pb. Cl 2+Mg(NO 3)2 v=0. 2 L v=0. 3 L c=0. 100 M c=0. 200 M n=? Find Limiting Reagent Pb(NO 3)2 : Pb. Cl 2 Mg. Cl 2 : Pb. Cl 2 1 : 1 0. 02: x 0. 06: x x=0. 02 mol of Pb. Cl 2 x=0. 06 mol of Pb. Cl 2 Note: Make sure to ALWAYS convert milliliters into liters for volume because molarity is always in units of moles per liter (mol/L) Limiting reagent is Pb(NO 3)2 Since the mole ratio between Pb(NO 3)2 : Pb. Cl 2 is 1: 1, the moles of Pb. Cl 2 is 0. 02 moles.

Ammonium sulfate is manufactured by reacting sulfuric acid with ammonia. What concentration of sulfuric acid is needed to react with 24. 4 m. L of a 2. 20 mol/L ammonia solution if 50. 0 m. L of sulfuric acid is used?

Answer: 0. 5368 M 1. Balance the equation: H 2 SO 4(aq) + 2 NH 3(aq) (NH 4)2(aq) + SO 4(aq) 2. What is given? c=2. 20 M n=c*v V=0. 0500 L n=? v= 0. 0244 L n=? n=2. 2 M*0. 0244 L n=0. 05368 moles 3. Solve for number of moles in ammonia. 4. Use mole ratio to find the number of moles of sulfuric acid H 2 SO 4 : NH 3 1: 2 x: 0. 05368 moles x= 0. 05368/2 x=0. 02684 moles H 2 SO 4 5. Find the concentration of sulfuric acid. c=n/v c=0. 02684 moles/0. 0500 L c=0. 5368 M Note: Make sure to ALWAYS convert milliliters into liters for volume because molarity is always in units of moles per liter (mol/L)

Calcium hydroxide is sometimes used in water treatment plants to clarify water for residential use. Calculate the volume of 0. 0250 mol/L calcium hydroxide solution that can be completely reacted with 25. 0 m. L of 0. 125 mol/L aluminum sulfate solution.
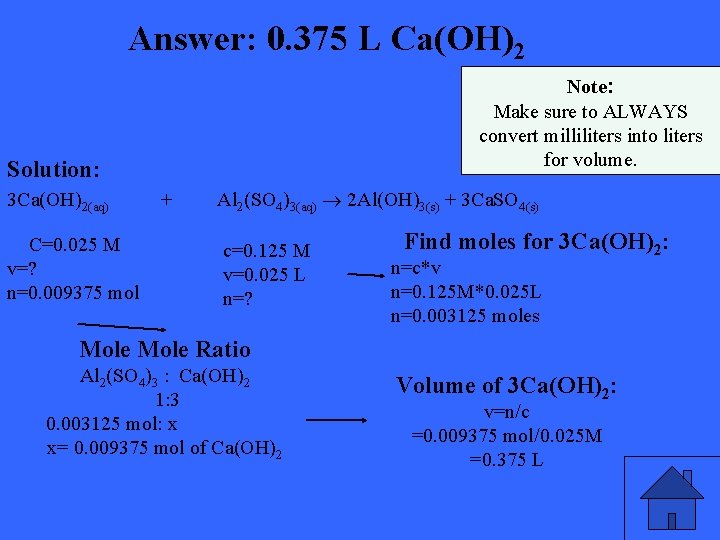
Answer: 0. 375 L Ca(OH)2 Note: Make sure to ALWAYS convert milliliters into liters for volume. Solution: 3 Ca(OH)2(aq) C=0. 025 M v=? n=0. 009375 mol + Al 2(SO 4)3(aq) 2 Al(OH)3(s) + 3 Ca. SO 4(s) c=0. 125 M v=0. 025 L n=? Find moles for 3 Ca(OH)2: n=c*v n=0. 125 M*0. 025 L n=0. 003125 moles Mole Ratio Al 2(SO 4)3 : Ca(OH)2 1: 3 0. 003125 mol: x x= 0. 009375 mol of Ca(OH)2 Volume of 3 Ca(OH)2: v=n/c =0. 009375 mol/0. 025 M =0. 375 L

Mr. Krstovic wants 75. 0 m. L of 0. 200 mol/L iron(III) chloride solution to react completely with an excess of 0. 250 mol/L sodium carbonate solution. What volume of sodium carbonate solution is needed?
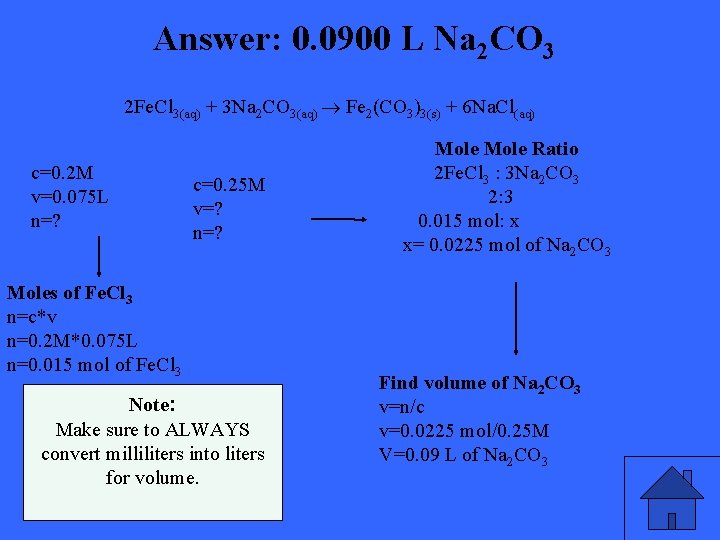
Answer: 0. 0900 L Na 2 CO 3 2 Fe. Cl 3(aq) + 3 Na 2 CO 3(aq) Fe 2(CO 3)3(s) + 6 Na. Cl(aq) c=0. 2 M v=0. 075 L n=? c=0. 25 M v=? n=? Moles of Fe. Cl 3 n=c*v n=0. 2 M*0. 075 L n=0. 015 mol of Fe. Cl 3 Note: Make sure to ALWAYS convert milliliters into liters for volume. Mole Ratio 2 Fe. Cl 3 : 3 Na 2 CO 3 2: 3 0. 015 mol: x x= 0. 0225 mol of Na 2 CO 3 Find volume of Na 2 CO 3 v=n/c v=0. 0225 mol/0. 25 M V=0. 09 L of Na 2 CO 3

Describe how you would go about separating a mixture containing solutions of strontium ions, Sr 2+(aq), and calcium ions, Ca 2+(aq).
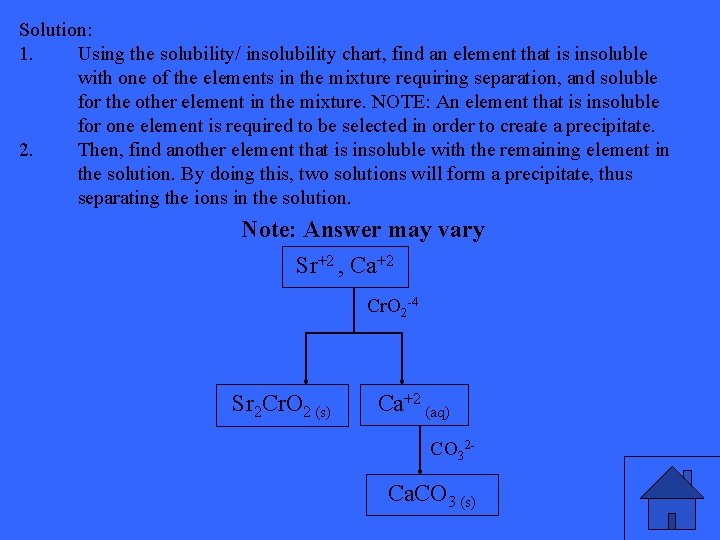
Solution: 1. Using the solubility/ insolubility chart, find an element that is insoluble with one of the elements in the mixture requiring separation, and soluble for the other element in the mixture. NOTE: An element that is insoluble for one element is required to be selected in order to create a precipitate. 2. Then, find another element that is insoluble with the remaining element in the solution. By doing this, two solutions will form a precipitate, thus separating the ions in the solution. Note: Answer may vary Sr+2 , Ca+2 Cr. O 2 -4 Sr 2 Cr. O 2 (s) Ca+2 (aq) CO 32 - Ca. CO 3 (s)

Using the flow chart method, separate the following cations from the aqueous solution: Ag+, Pb 2+, Ba 2+, Cu+2, Fe 3+
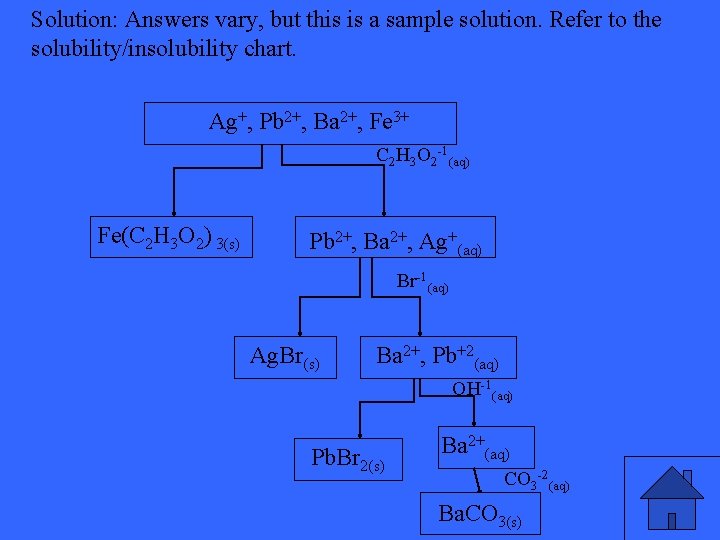
Solution: Answers vary, but this is a sample solution. Refer to the solubility/insolubility chart. Ag+, Pb 2+, Ba 2+, Fe 3+ C 2 H 3 O 2 -1(aq) Fe(C 2 H 3 O 2) 3(s) Pb 2+, Ba 2+, Ag+(aq) Br-1(aq) Ag. Br(s) Ba 2+, Pb+2(aq) OH-1(aq) Pb. Br 2(s) Ba 2+(aq) CO 3 -2(aq) Ba. CO 3(s)

Using the flow chart method, separate the following cations from the aqueous solution: Br-, Cl. O 3 -2, O-2, NO 3 -
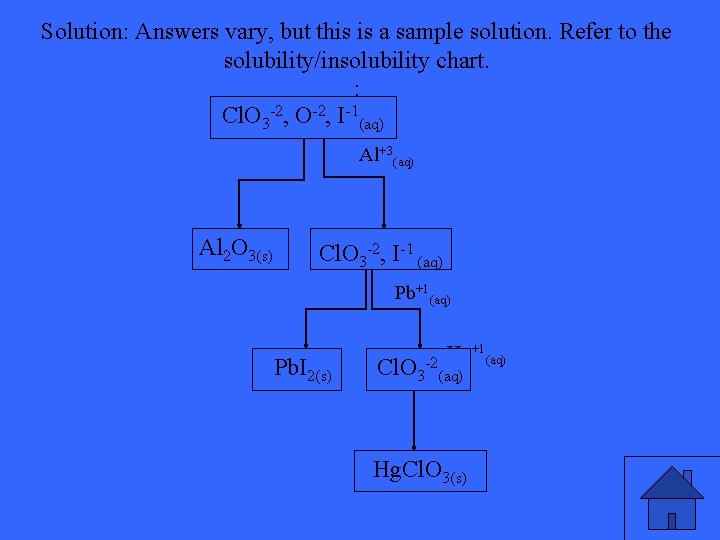
Solution: Answers vary, but this is a sample solution. Refer to the solubility/insolubility chart. : Cl. O 3 -2, O-2, I-1(aq) Al+3(aq) Al 2 O 3(s) Cl. O 3 -2, I-1 (aq) Pb+1(aq) Pb. I 2(s) Hg+1(aq) Cl. O 3 -2(aq) Hg. Cl. O 3(s)

Write the total and net ionic equation for the reaction Lead (II) nitrate with hydrochloric acid.

Answer: Total Ionic Equation: Pb 2+(aq) + 2 NO 3‾ (aq) + 2 H+(aq) + 2 Cl‾ (aq) Pb. Cl 2 (s) + 2 H+(aq) + 2 NO 3‾ (aq) Net Ionic Equation: Pb 2+(aq) + 2 Cl‾ (aq) Pb. Cl 2 (s) Solution: Finding the total ionic equation: 1. Write a balanced reaction from the information provided. : Pb(NO 3)2 (aq) + 2 HCl (aq) Pb. Cl 2 (s) + 2 HNO 3 (aq) 2. Write the elemental composition for all AQUEOUS compound. For solids, do not dissociate. Pb 2+(aq) + 2 NO 3‾ (aq) + 2 H+(aq) + 2 Cl‾ (aq) Pb. Cl 2 (s) + 2 H+(aq) + 2 NO 3‾ (aq) 3. Step 2 is the total ionic equation Finding the net ionic equation: 1. Cancel out elements that have the same charge Pb 2+(aq) + 2 NO 3‾ (aq) + 2 H+(aq) + 2 Cl‾ (aq) Pb. Cl 2 (s) + 2 H+(aq) + 2 NO 3‾ (aq) 2. The net ionic equation is the elements from the reactants that do are left over after completing step 1. The remaining reactants should correspond to the product. Pb 2+(aq) + 2 Cl‾ (aq) Pb. Cl 2 (s)

What will be the complete, total ionic and net ionic equations for the reaction of an aqueous solution of nitric acid (HNO 3) with one of potassium hydroxide (KOH)?
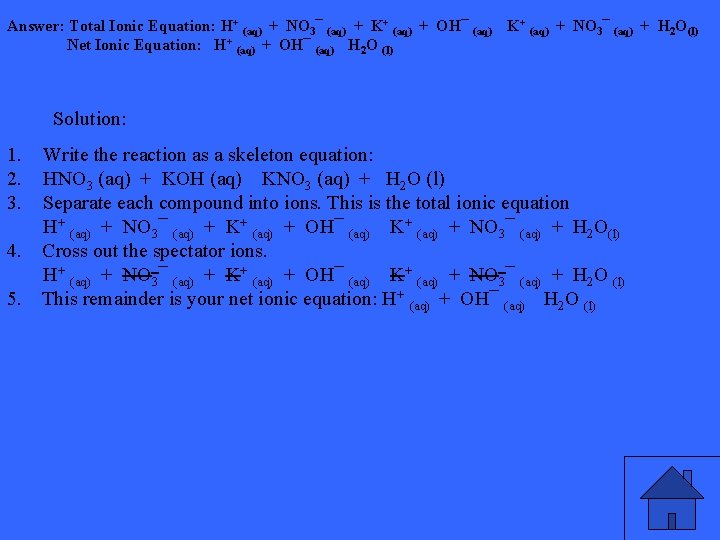
Answer: Total Ionic Equation: H+ (aq) + NO 3¯ (aq) + K+ (aq) + OH¯ (aq) K+ (aq) + NO 3¯ (aq) + H 2 O(l) Net Ionic Equation: H+ (aq) + OH¯ (aq) H 2 O (l) Solution: 1. 2. 3. Write the reaction as a skeleton equation: HNO 3 (aq) + KOH (aq) KNO 3 (aq) + H 2 O (l) Separate each compound into ions. This is the total ionic equation H+ (aq) + NO 3¯ (aq) + K+ (aq) + OH¯ (aq) K+ (aq) + NO 3¯ (aq) + H 2 O(l) 4. Cross out the spectator ions. H+ (aq) + NO 3¯ (aq) + K+ (aq) + OH¯ (aq) K+ (aq) + NO 3¯ (aq) + H 2 O (l) 5. This remainder is your net ionic equation: H+ (aq) + OH¯ (aq) H 2 O (l)
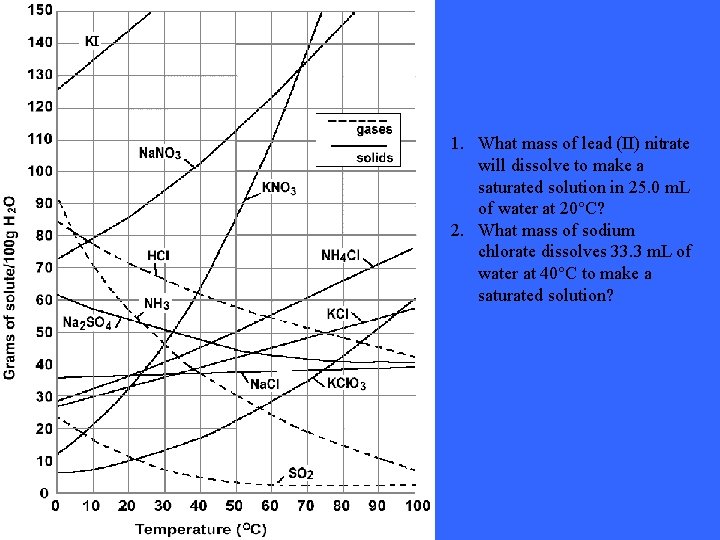
1. What mass of lead (II) nitrate will dissolve to make a saturated solution in 25. 0 m. L of water at 20°C? 2. What mass of sodium chlorate dissolves 33. 3 m. L of water at 40°C to make a saturated solution?

Solutions: 1. 2. 132. 5 grams, assuming 53 g/100 m. L of water dissolves 38. 33 grams assuming 155 g/100 m. L dissolves These answers and questions were taken from the following website: http: //74. 125. 93. 132/search? q=cache: 07 X_n 6 d 17 SQJ: www. pembinat rails. ca/shaftesbury/mrdeakin/Solubility%2520 Curve%2520 Question s. 052. doc+solubility+curve+questions&cd=1&hl=en&ct=clnk&gl=ca &client=firefox-a Still not understanding the concept of solubility curves? Take a look at this site: http: //www. thesciencedesk. com/sgsolubilitygraph. htm
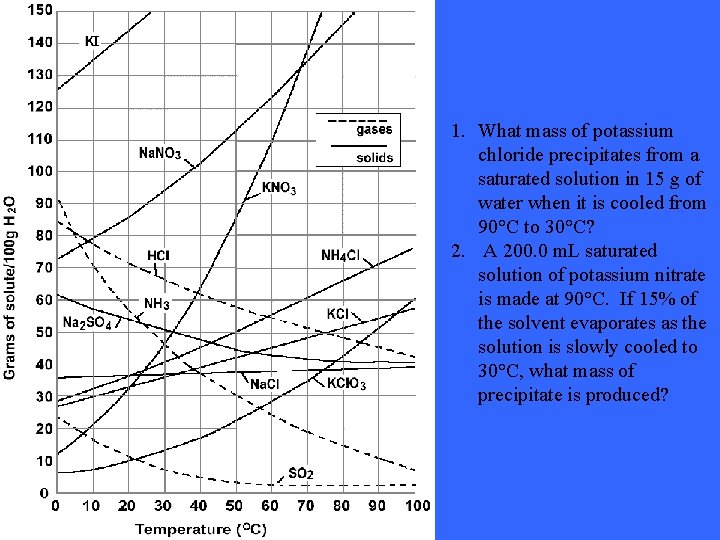
1. What mass of potassium chloride precipitates from a saturated solution in 15 g of water when it is cooled from 90°C to 30°C? 2. A 200. 0 m. L saturated solution of potassium nitrate is made at 90°C. If 15% of the solvent evaporates as the solution is slowly cooled to 30°C, what mass of precipitate is produced?

Solutions: 1. 2 grams with 36 grams/100 m. L at 90 degrees Celsius, 28 grams / 100 m. L at 30 degrees Celsius 325. 1 grams with 202. 5 grams at 90 degrees Celsius , 47 grams at 30 degrees Celsius. These answers and questions were taken from the following website: http: //74. 125. 93. 132/search? q=cache: 07 X_n 6 d 17 SQJ: www. pembinatr ails. ca/shaftesbury/mrdeakin/Solubility%2520 Curve%2520 Questions. 052. doc+solubility+curve+questions&cd=1&hl=en&ct=clnk&gl=ca& client=firefox-a Still not understanding the concept of solubility curves? Take a look at this site: http: //www. thesciencedesk. com/sgsolubilitygraph. htm
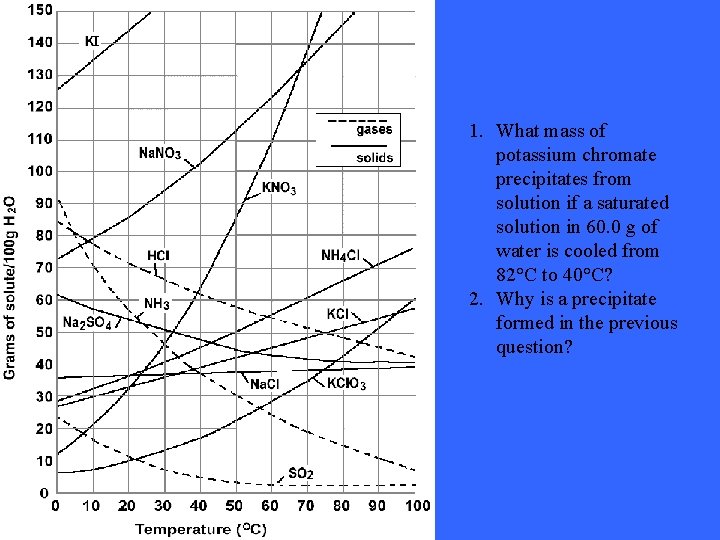
1. What mass of potassium chromate precipitates from solution if a saturated solution in 60. 0 g of water is cooled from 82°C to 40°C? 2. Why is a precipitate formed in the previous question?

Solutions: 1. 2. 4. 8 grams with 74 grams/100 m. L at 40 degrees Celsius Solubility of ionic solids decrease with temperatures These answers and questions were taken from the following website: http: //74. 125. 93. 132/search? q=cache: 07 X_n 6 d 17 SQJ: www. pembinatra ils. ca/shaftesbury/mrdeakin/Solubility%2520 Curve%2520 Questions. 05 2. doc+solubility+curve+questions&cd=1&hl=en&ct=clnk&gl=ca&clie nt=firefox-a Still not understanding the concept of solubility curves? Take a look at this site: http: //www. thesciencedesk. com/sgsolubilitygraph. htm
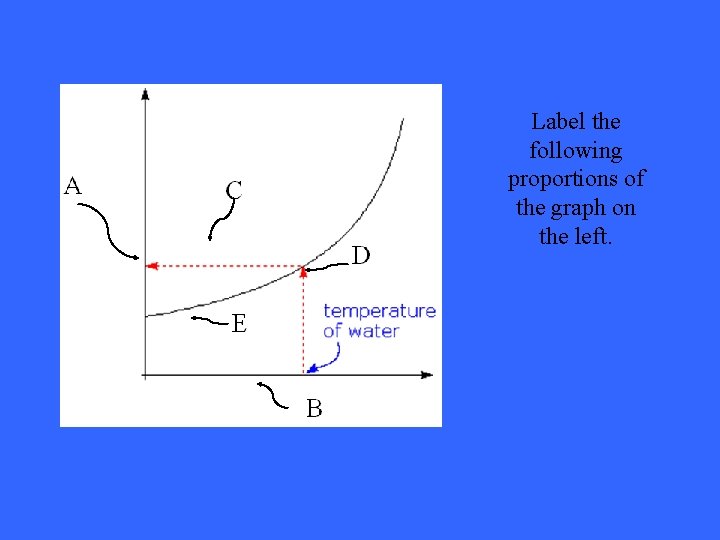
Label the following proportions of the graph on the left.
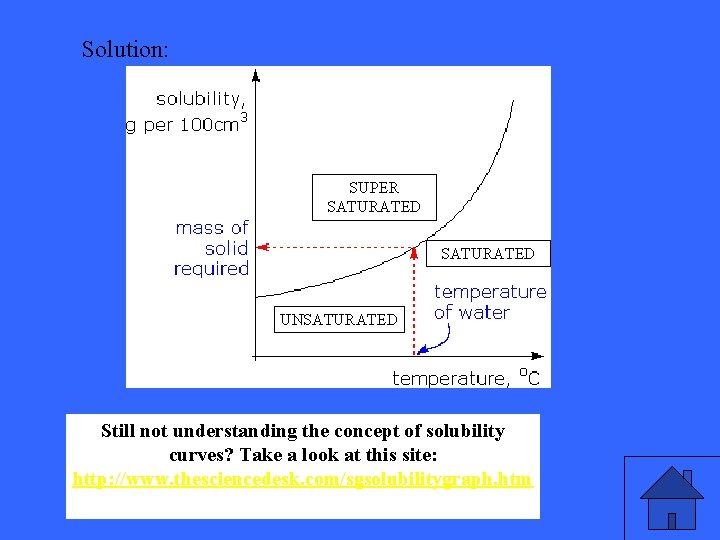
Solution: SUPER SATURATED UNSATURATED Still not understanding the concept of solubility curves? Still not understanding the concept of solubility Take a look at this site: curves? Take a look at this site: http: //www. thesciencedesk. com/sgsolubilitygraph. htm
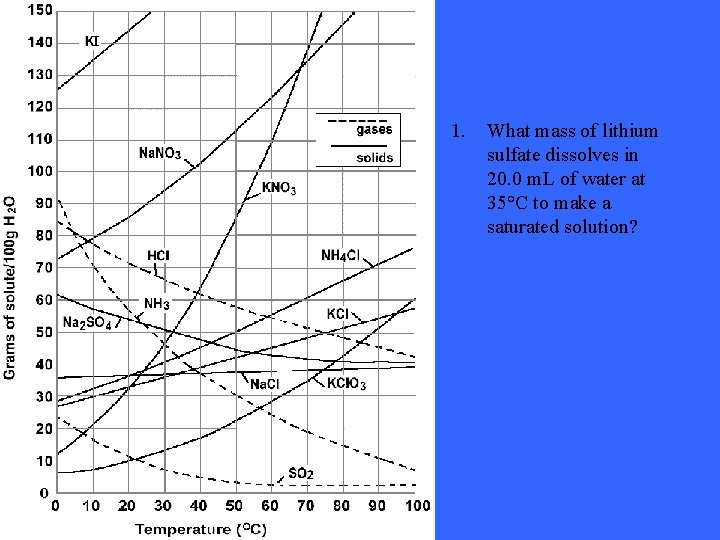
1. What mass of lithium sulfate dissolves in 20. 0 m. L of water at 35°C to make a saturated solution?

Solution: 1. 7 grams assuming 35 grams/100 m. L water dissolves These answers and questions were taken from the following website: http: //74. 125. 93. 132/search? q=cache: 07 X_n 6 d 17 SQJ: www. pembin atrails. ca/shaftesbury/mrdeakin/Solubility%2520 Curve%2520 Questi ons. 052. doc+solubility+curve+questions&cd=1&hl=en&ct=clnk&gl =ca&client=firefox-a Still not understanding the concept of solubility curves? Take a look at this site: http: //www. thesciencedesk. com/sgsolubilitygraph. htm

20. 0 m. L of a 3. 0 M HCl solution are mixed with 20. 0 m. L of a 5. 0 M Na. OH solution. Is the p. H of the resulting solution above 7, below 7, or 7? Explain your answer.

Solution found on the following VIDEO (Very DETAILED!!): http: //www. youtube. com/watch? v=IX 9 RHZDVb. YM&f eature=related

The molarity of a hydrochloric acid solution can be determined by titrating a known volume of the solution with a sodium hydroxide solution of known concentration. If 14. 7 m. L of 0. 102 M Na. OH is required to titrate 25. 00 m. L of a hydrochloric acid, HCl, solution, what is the molarity of the hydrochloric acid?

Answer: 0. 0579 M HCl Solution: 1. Write a balanced equation: HCl + Na. OH H 2 O+ Na. Cl 2. Write down the given values: v=0. 025 L v=0. 0147 L Number of Moles Na. OH: c= ? c= 0. 102 M n=(0. 0147 L)(0. 102 M) n=? n=0. 0014994 moles Na. OH 3. Solve for number of moles of Na. OH and then use mole-mole ratio to solve for the moles of HCl. 4. Find Number of Moles of HCl: Moles of HCl : Moles of Na. OH 1 : 1 x : 0. 0014994 Therefore, since mole ratio is 1: 1, the moles of HCl is also 0. 0014994 Calculate the concentration of HCl. c=n/v c=(0. 0014994 moles)/0. 025 L c=0. 0579 M
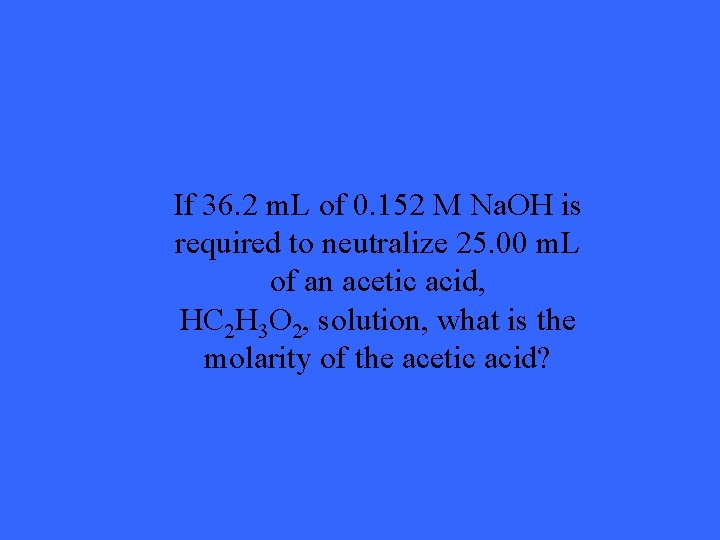
If 36. 2 m. L of 0. 152 M Na. OH is required to neutralize 25. 00 m. L of an acetic acid, HC 2 H 3 O 2, solution, what is the molarity of the acetic acid?

Answer: 0. 220 M HC 2 H 3 O 2 Solution: 1. Write a balanced equation: HC 2 H 3 O 2 + Na. OH H 2 O + Na. C 2 H 3 O 2 2. Write down the given values: v=0. 025 L Number of Moles Na. OH: v=0. 0362 L c= ? n=(0. 0362 L)(0. 152 M) c= 0. 152 M n=? n=0. 0055024 moles Na. OH n=? 3. Solve for number of moles of Na. OH and then use mole-mole ratio to solve for the moles of HCl. 4. Find Number of Moles of HCl: Moles of C 2 H 3 O 2 : Moles of HCl 1 : 1 x : 0. 0055024 Therefore, since mole ratio is 1: 1, the moles of HCl is also 0. 0055024 Calculate the concentration of HCl. c=n/v c=(0. 0055024 moles)/0. 025 L c=0. 220096 M

If 11. 3 m. L of 0. 110 M HCl is required to neutralize 25. 00 m. L of a barium hydroxide solution, what is the molarity of the barium hydroxide?
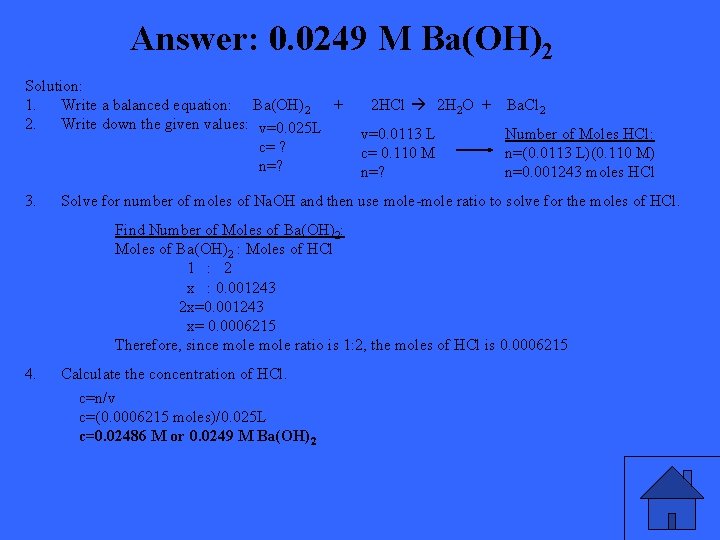
Answer: 0. 0249 M Ba(OH)2 Solution: 1. Write a balanced equation: Ba(OH)2 + 2 HCl 2 H 2 O + Ba. Cl 2 2. Write down the given values: v=0. 025 L v=0. 0113 L Number of Moles HCl: c= ? c= 0. 110 M n=(0. 0113 L)(0. 110 M) n=? n=0. 001243 moles HCl 3. Solve for number of moles of Na. OH and then use mole-mole ratio to solve for the moles of HCl. Find Number of Moles of Ba(OH)2: Moles of Ba(OH)2 : Moles of HCl 1 : 2 x : 0. 001243 2 x=0. 001243 x= 0. 0006215 Therefore, since mole ratio is 1: 2, the moles of HCl is 0. 0006215 4. Calculate the concentration of HCl. c=n/v c=(0. 0006215 moles)/0. 025 L c=0. 02486 M or 0. 0249 M Ba(OH)2

Complete equations for the following acids ionizing or dissociating in water: 1. (NH 4)3 PO 4 (s) → ? 2. Mg(OH)2 (s) → ? 3. H 2 SO 4 (g) + H 2 O(l)→ ? 4. HCl(g) + H 2 O(l) → ? Classify the acid, base, conjugate base and conjugate acid 5. NH 3 + H 2 O→ NH 4+ OH 6. H 2 SO 4 + H 2 O → HSO 4 - + H 3 O+ 7. HCl + OH- → Cl- + H 2 O 8. HCl + NH 3 → Cl- + NH 4+

Solutions: 1. (NH 4)3 PO 4 (s) → 3 NH 4+(aq) + PO 43 -(aq) 2. Mg(OH)2 (s) → Mg 2+(aq) + 2 OH– (aq) 3. H 2 SO 4 (g) + H 2 O(l)→ 2 H 3 O+(aq) + SO 42 -(aq) 4. HCl(g) + H 2 O(l) → H 3 O+(aq) + Cl-(aq) 5. NH 3 + H 2 O→ NH 4+ OH NH 3 = Base H 2 O= Acid NH 4= Conjugate Acid OH-1= Conjugate Base 6. H 2 SO 4 + H 2 O → HSO 4 - + H 3 O+ H 2 SO 4 = Acid H 2 O = Base HSO 4 - = Conjugate Acid H 3 O+ = Conjugate Base 7. HCl + OH- → Cl- + H 2 O HCl = Acid OH- = Base Cl- = Conjugate Acid H 2 O = Conjugate Base 8. HCl + NH 3 → Cl- + NH 4+ HCl = Acid NH 3 = Base Cl- = Conjugate Acid NH 4+ = Conjugate Base
- Slides: 51